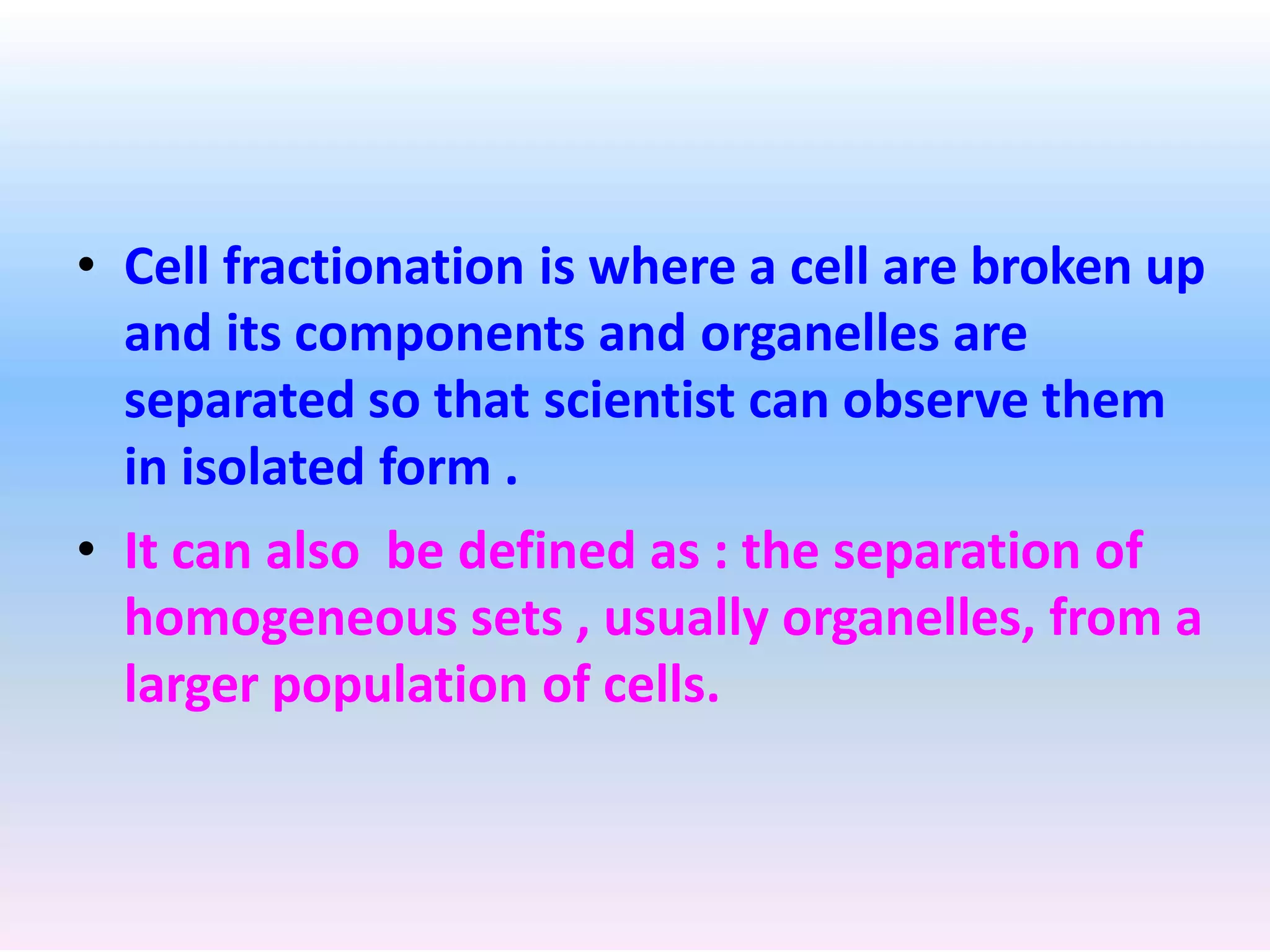
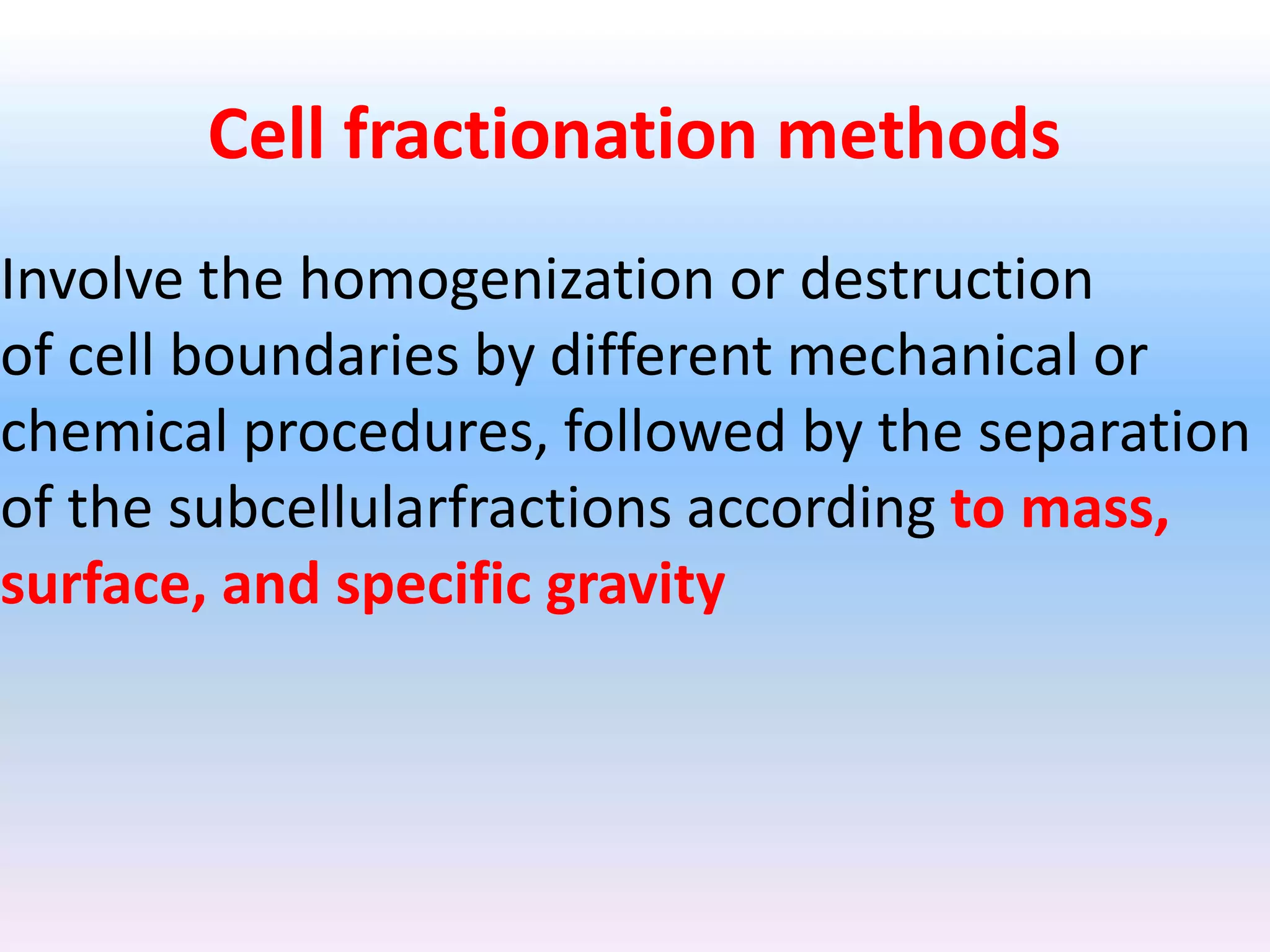
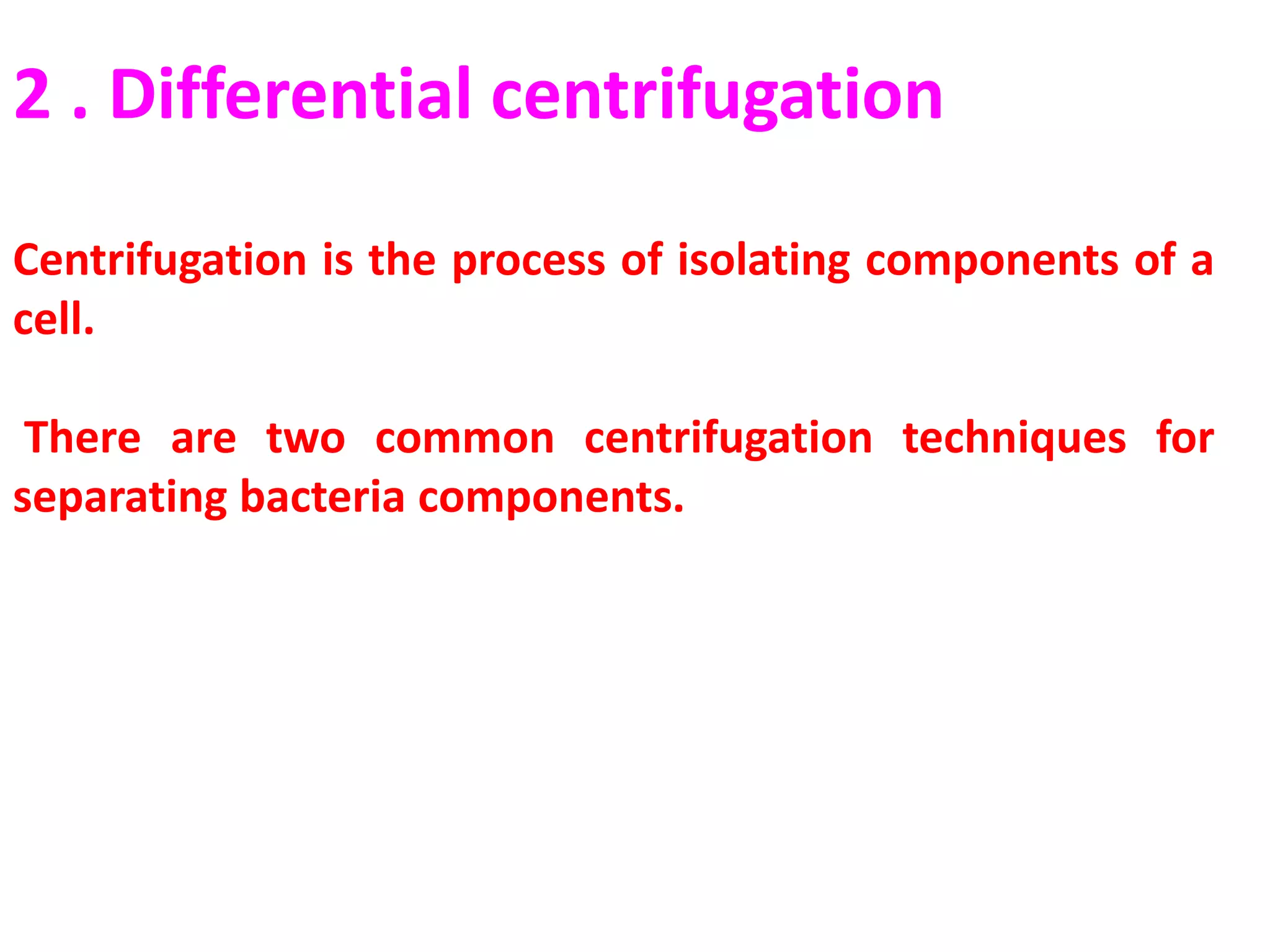
Cell fractionation is a technique used by biologists to isolate and study specific organelles from cells, involving procedures such as homogenization and differential centrifugation. This method has led to significant insights into cellular processes, including protein synthesis and energy conversion in mitochondria and chloroplasts. The document outlines steps for cell disruption, centrifugation methods, and analysis of separated fractions to advance understanding of organelle functions.