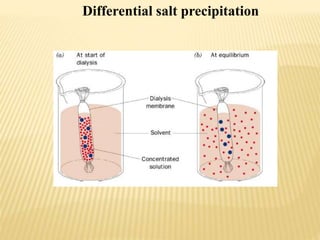
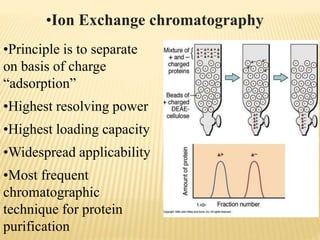
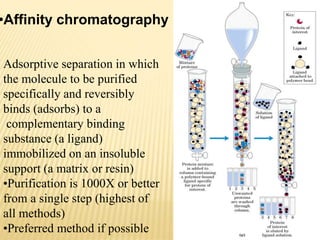
This document discusses protein purification techniques. It describes that proteins can be extracellular, secreted from cells, or intracellular, requiring cell disruption. Purification techniques include differential centrifugation to separate components by size and mass, differential salt precipitation to separate based on solubility, and various types of chromatography including size exclusion, ion exchange, and affinity chromatography. Electrophoresis can also separate proteins based on size using electrical current. The overall goal is to purify proteins for uses such as commercial products, research, or analysis of structure and function.