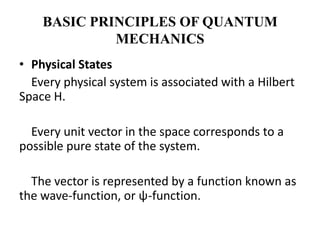
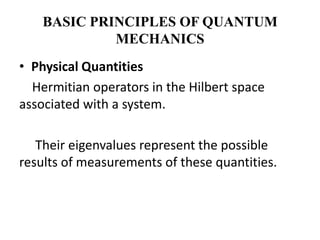
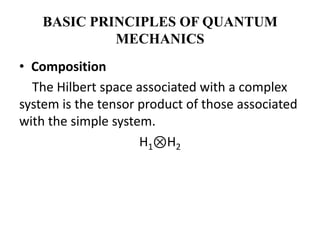
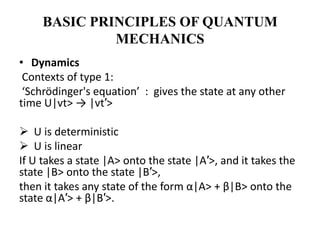
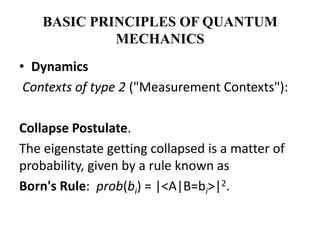
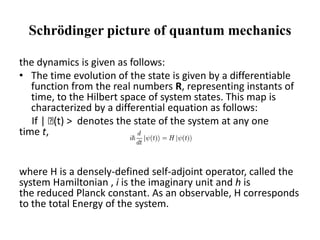
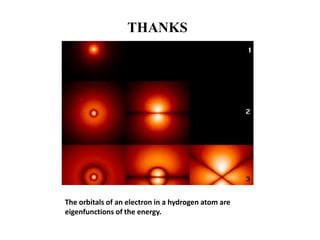
This document discusses the mathematical formulation of quantum mechanics. It describes how quantum systems are represented using linear algebra concepts such as Hilbert spaces and operators. Physical states are represented by unit vectors in a Hilbert space. Observables are represented by Hermitian operators whose eigenvalues correspond to possible measurement outcomes. Dynamics are governed by Schrodinger's equation, which describes how states evolve over time.