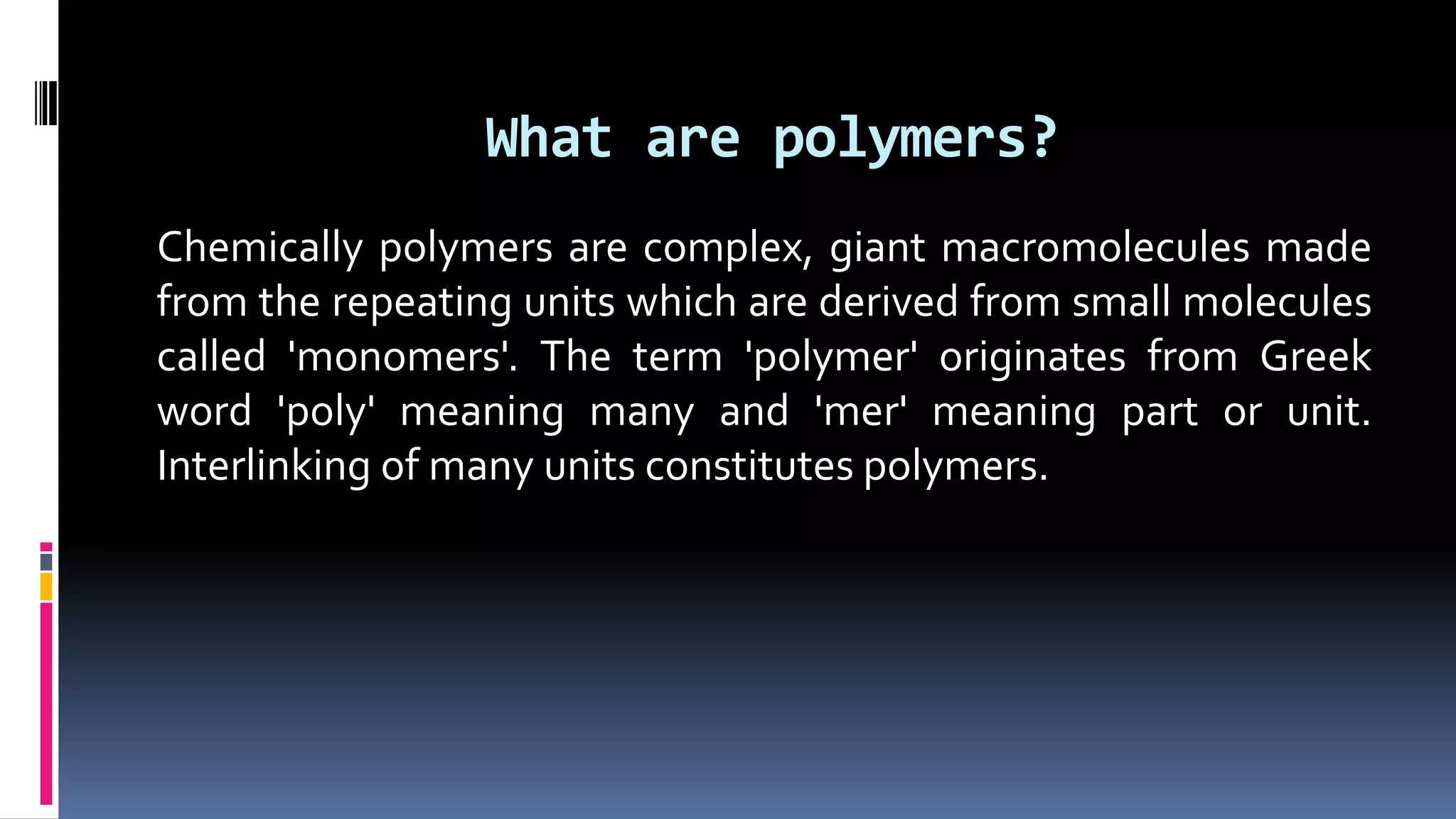
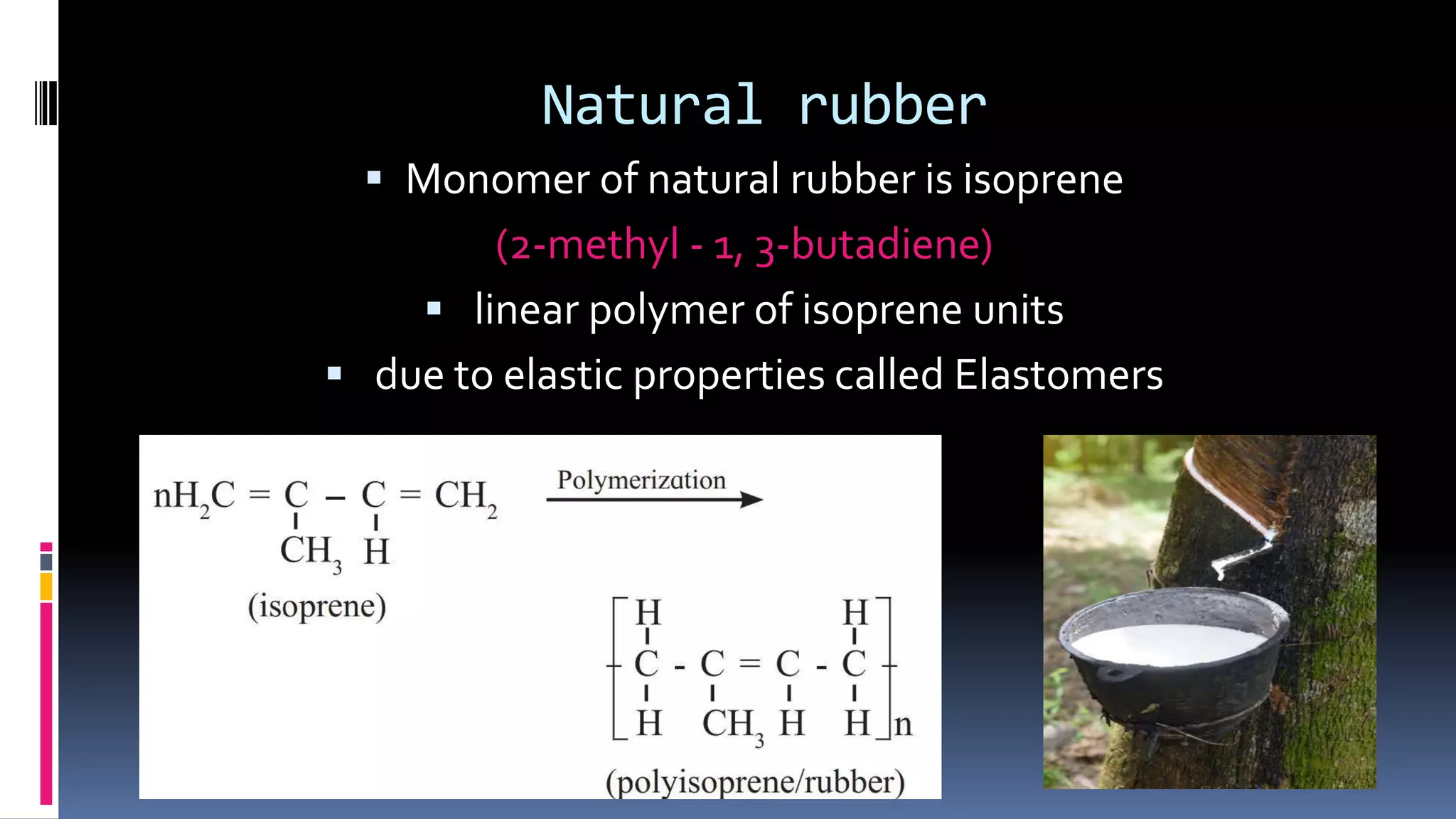
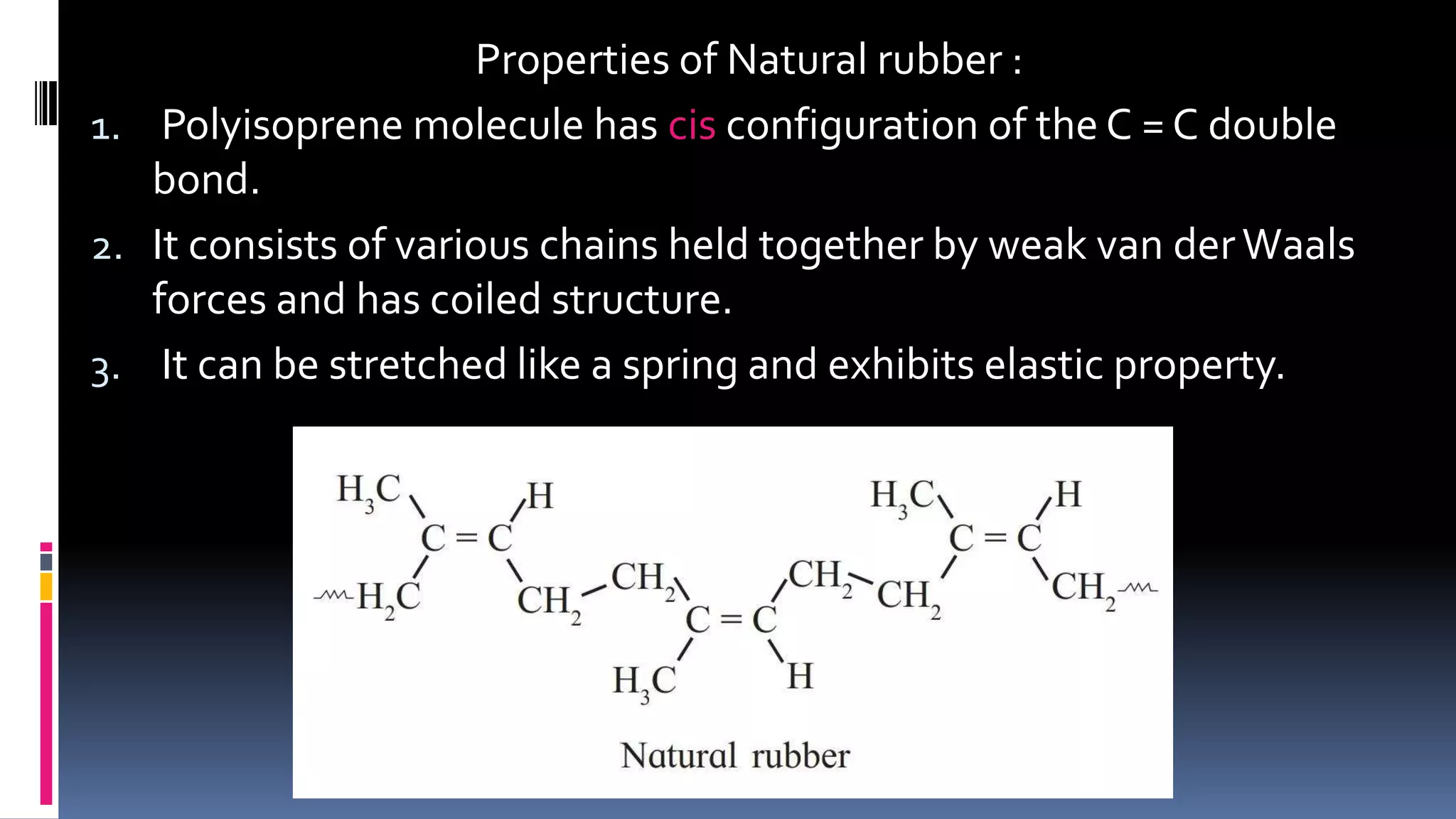
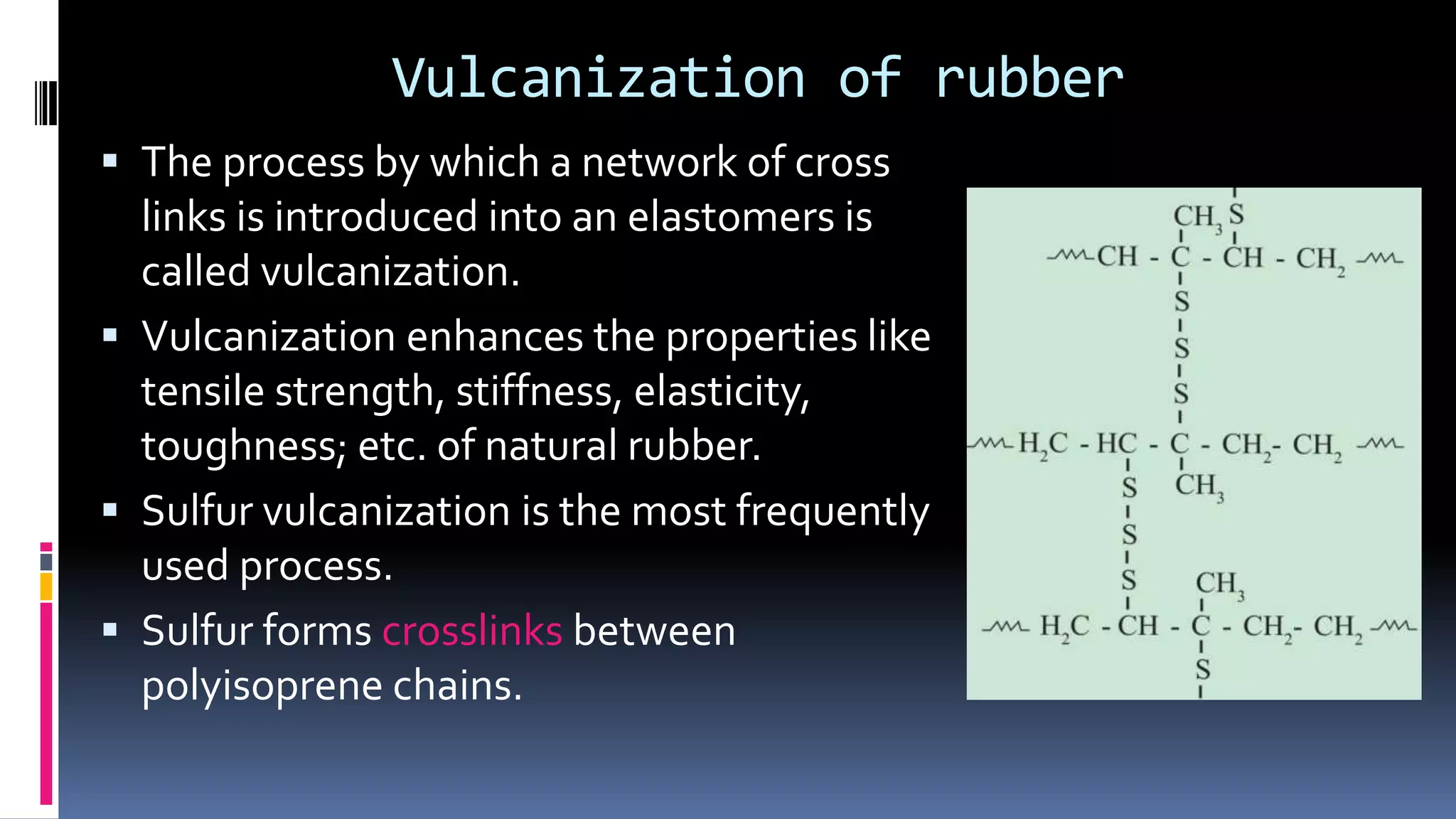
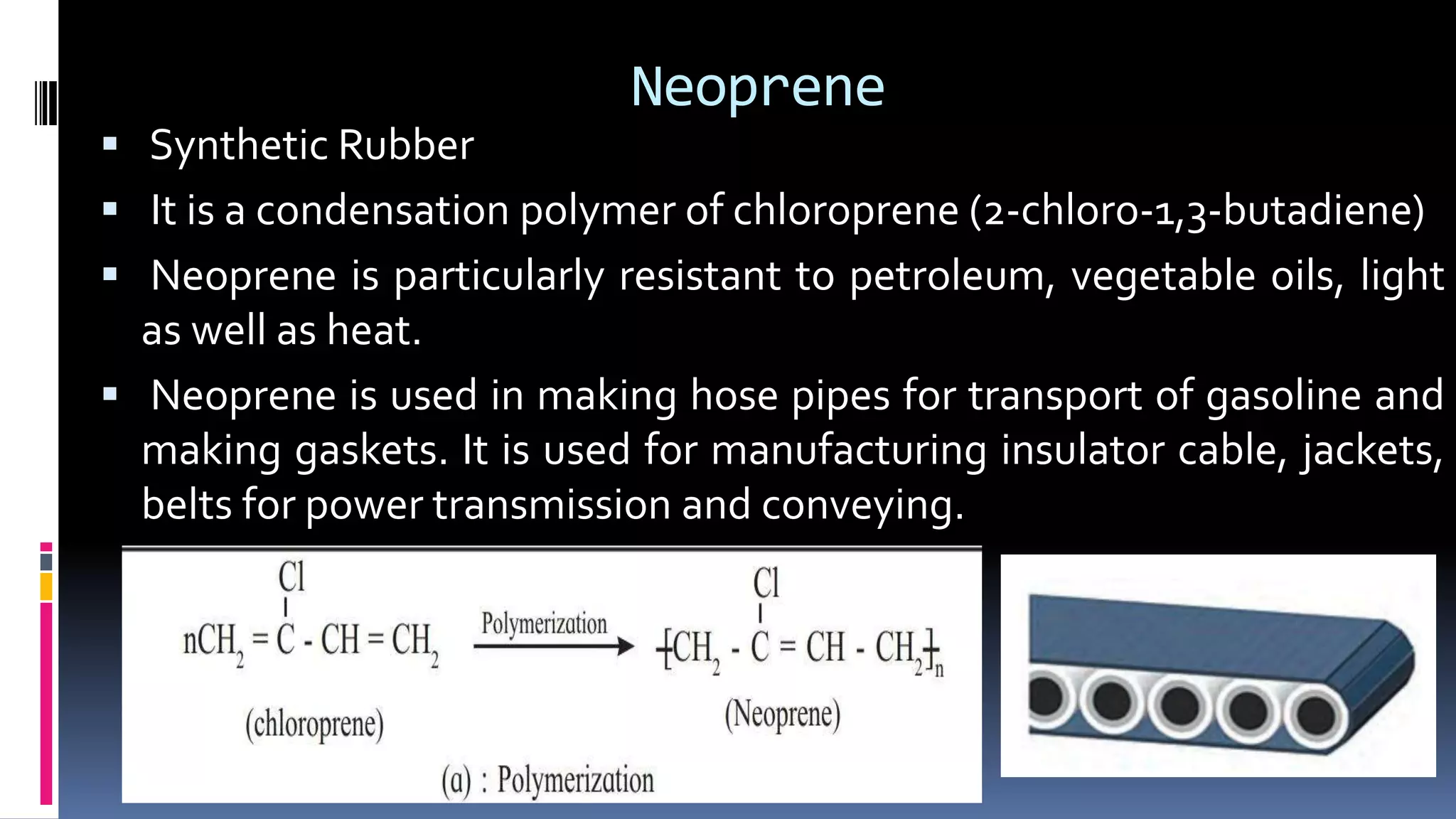
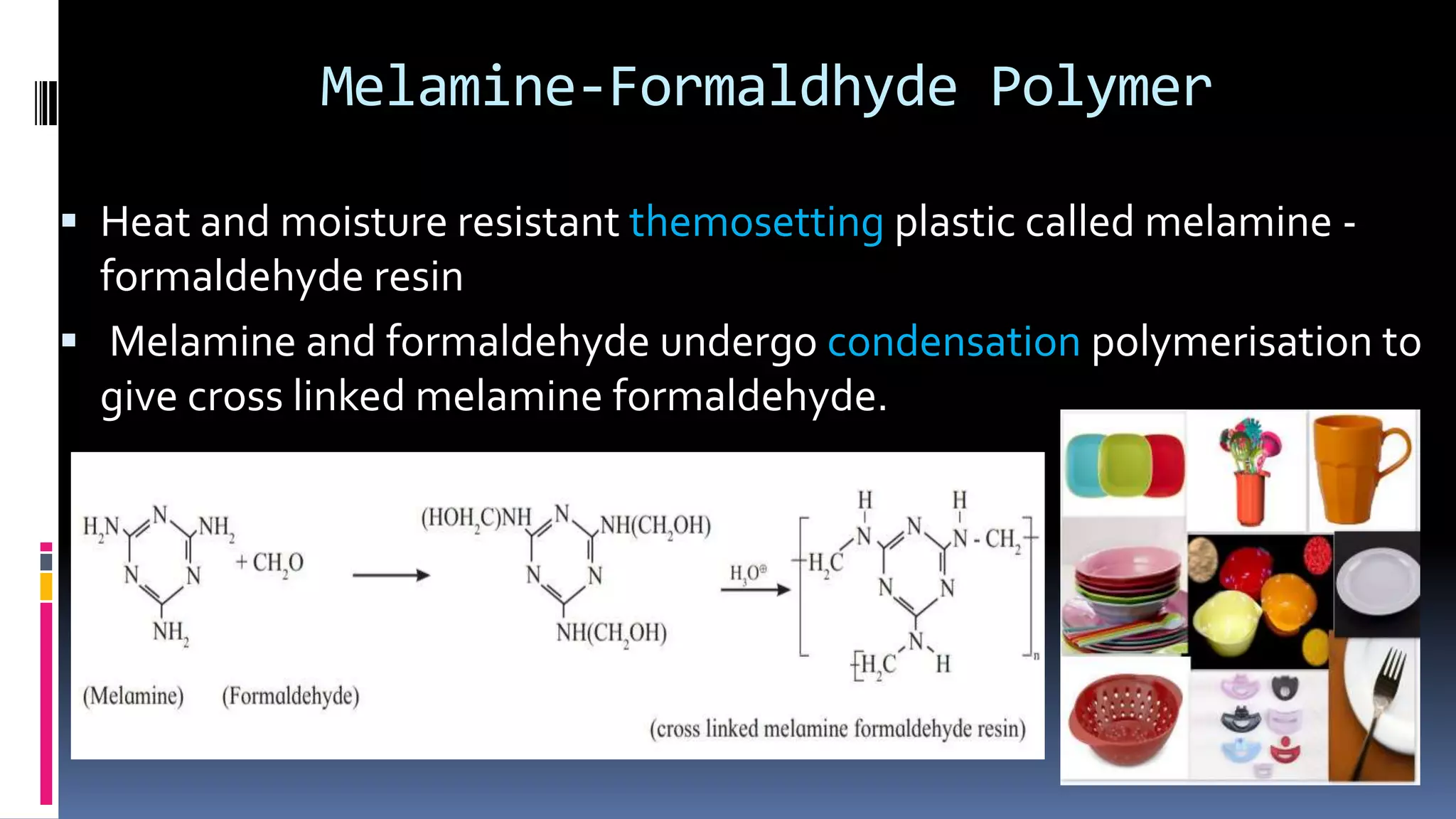
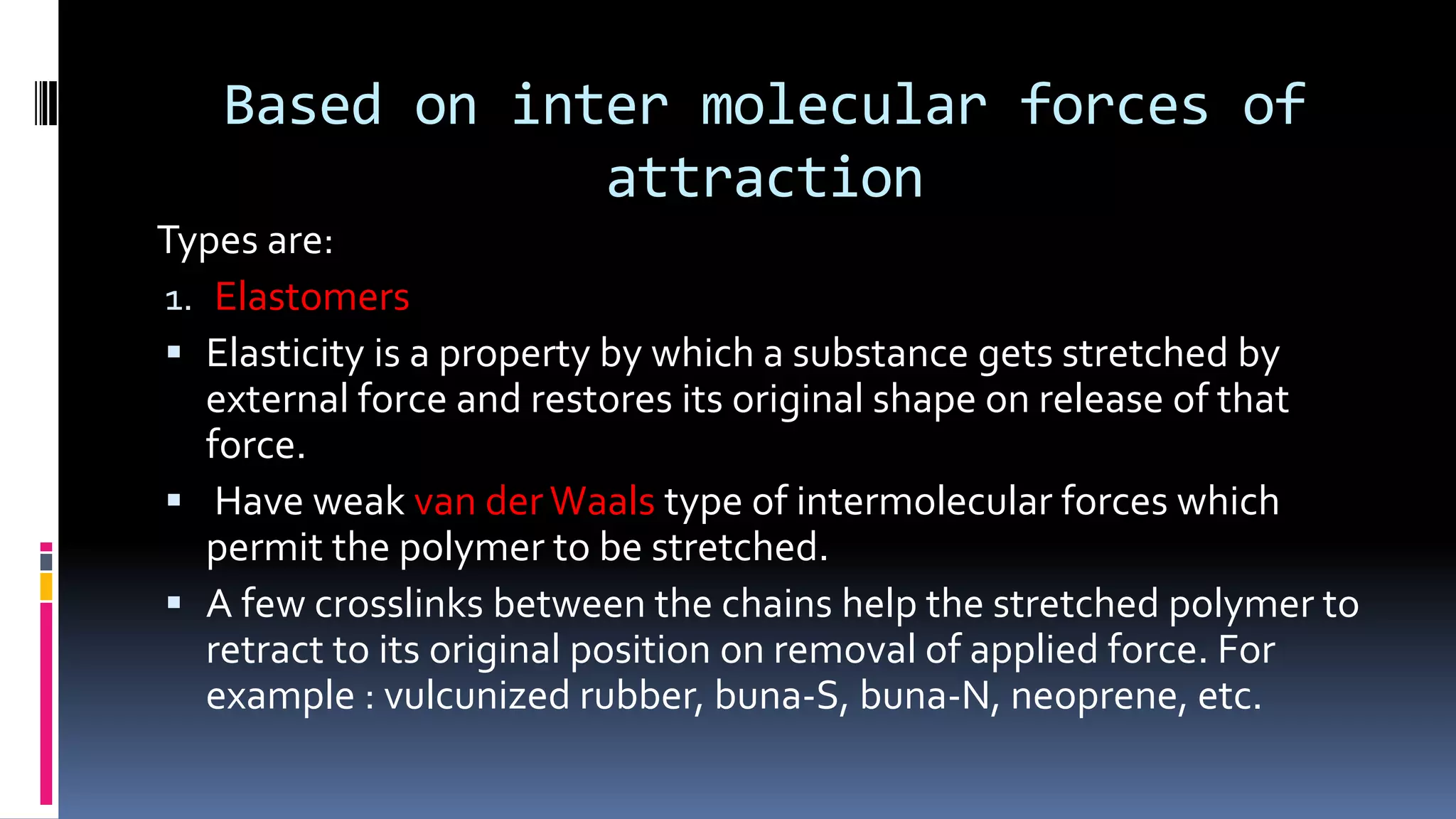
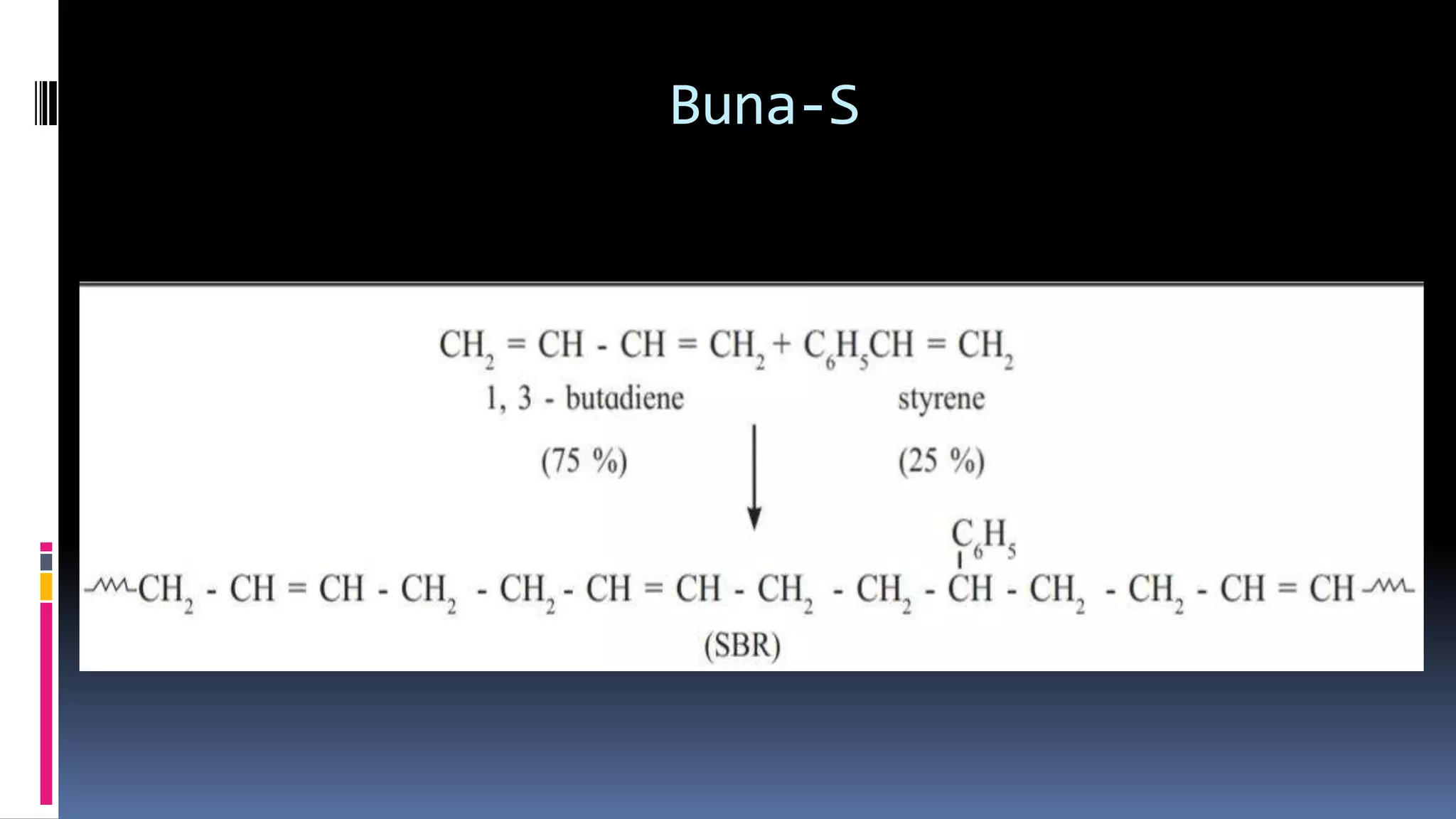
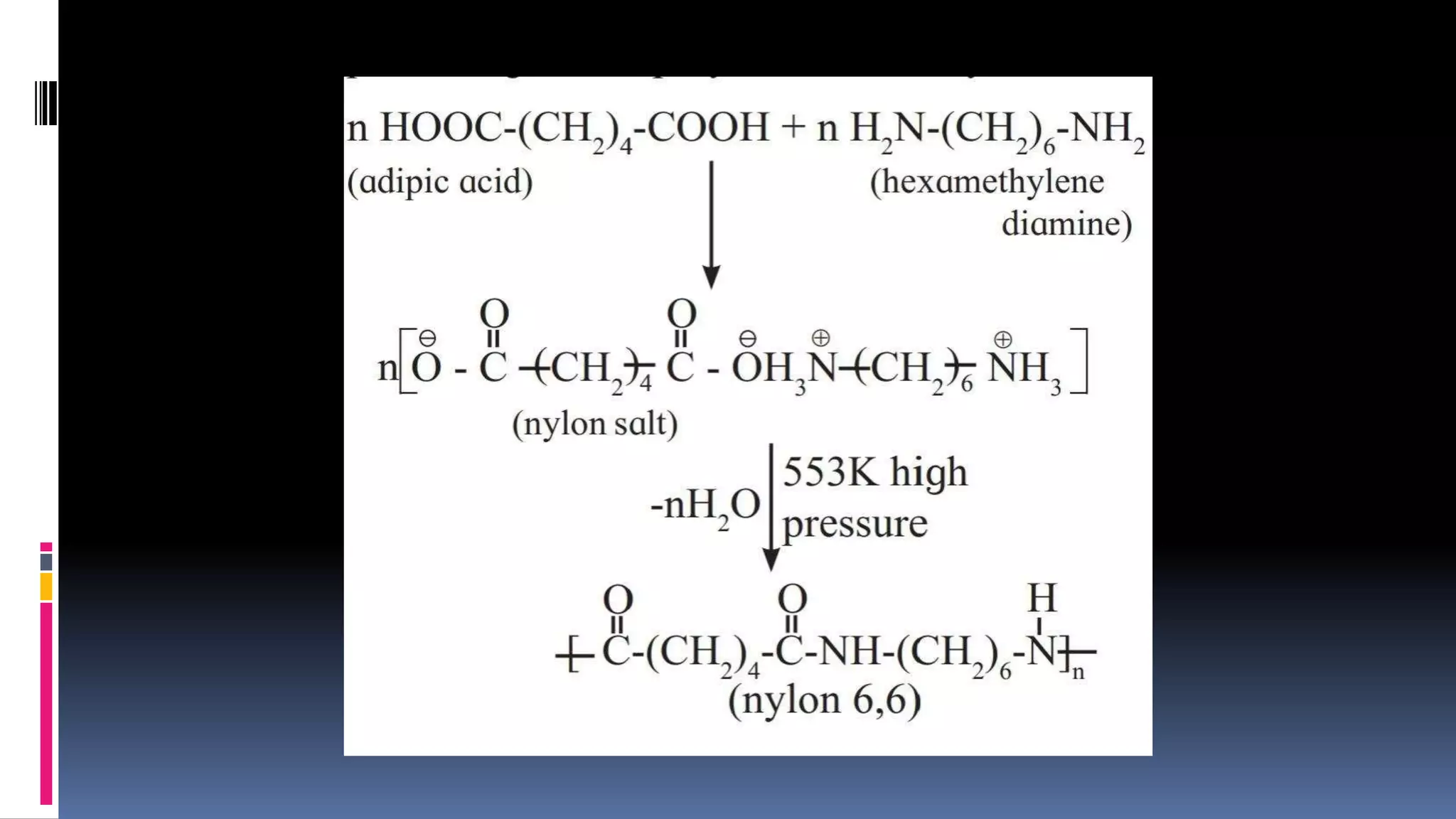
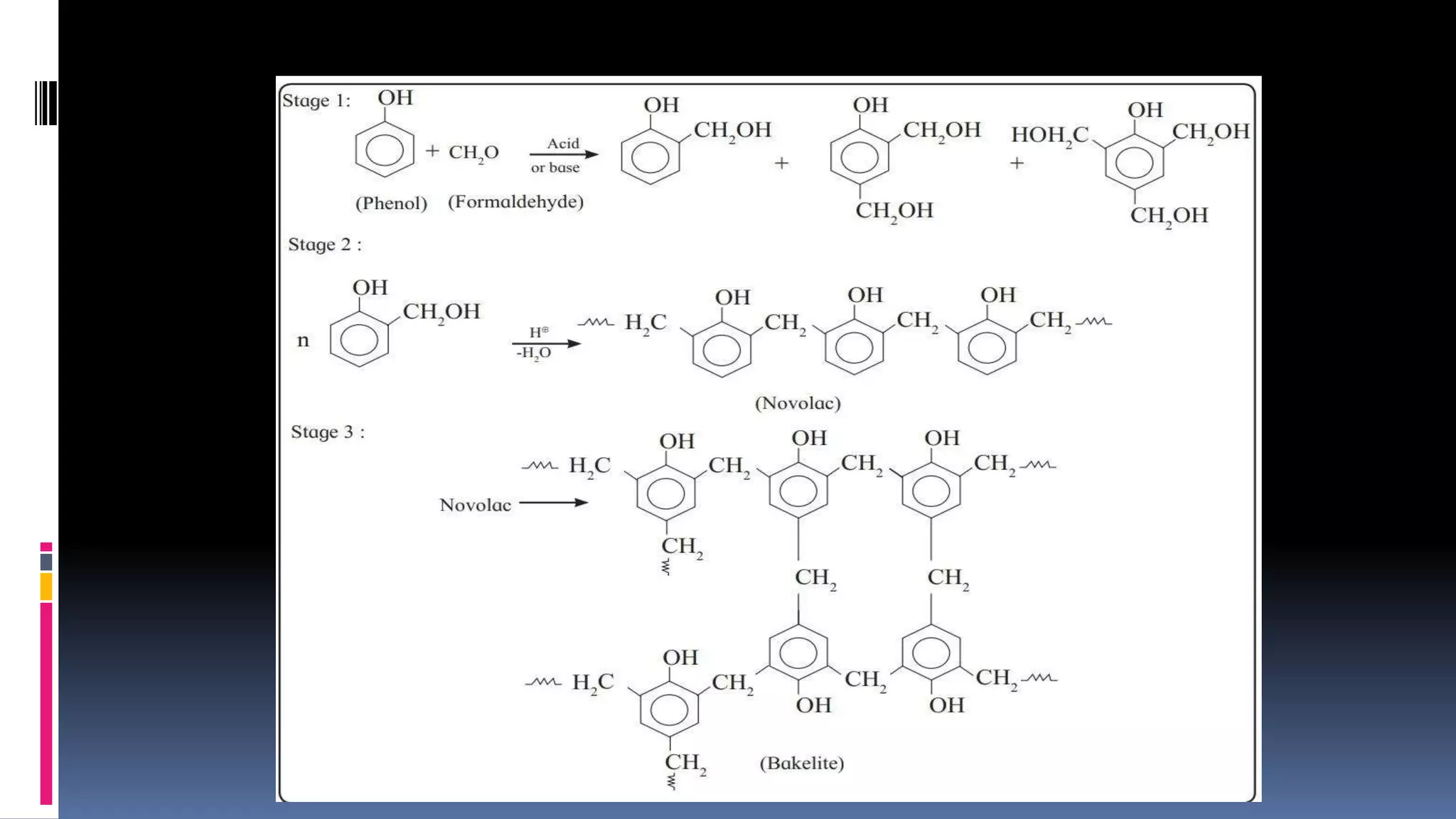
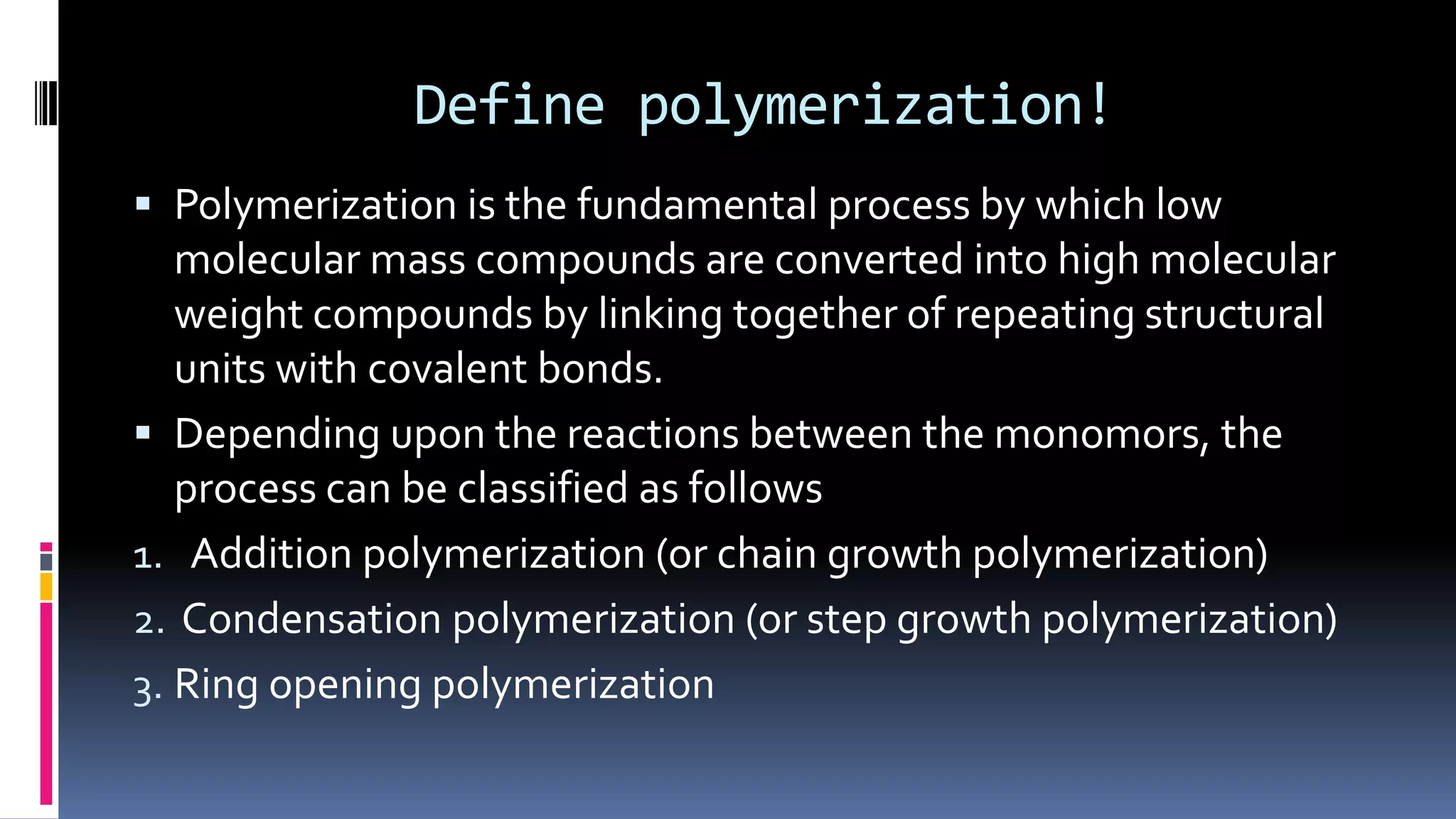
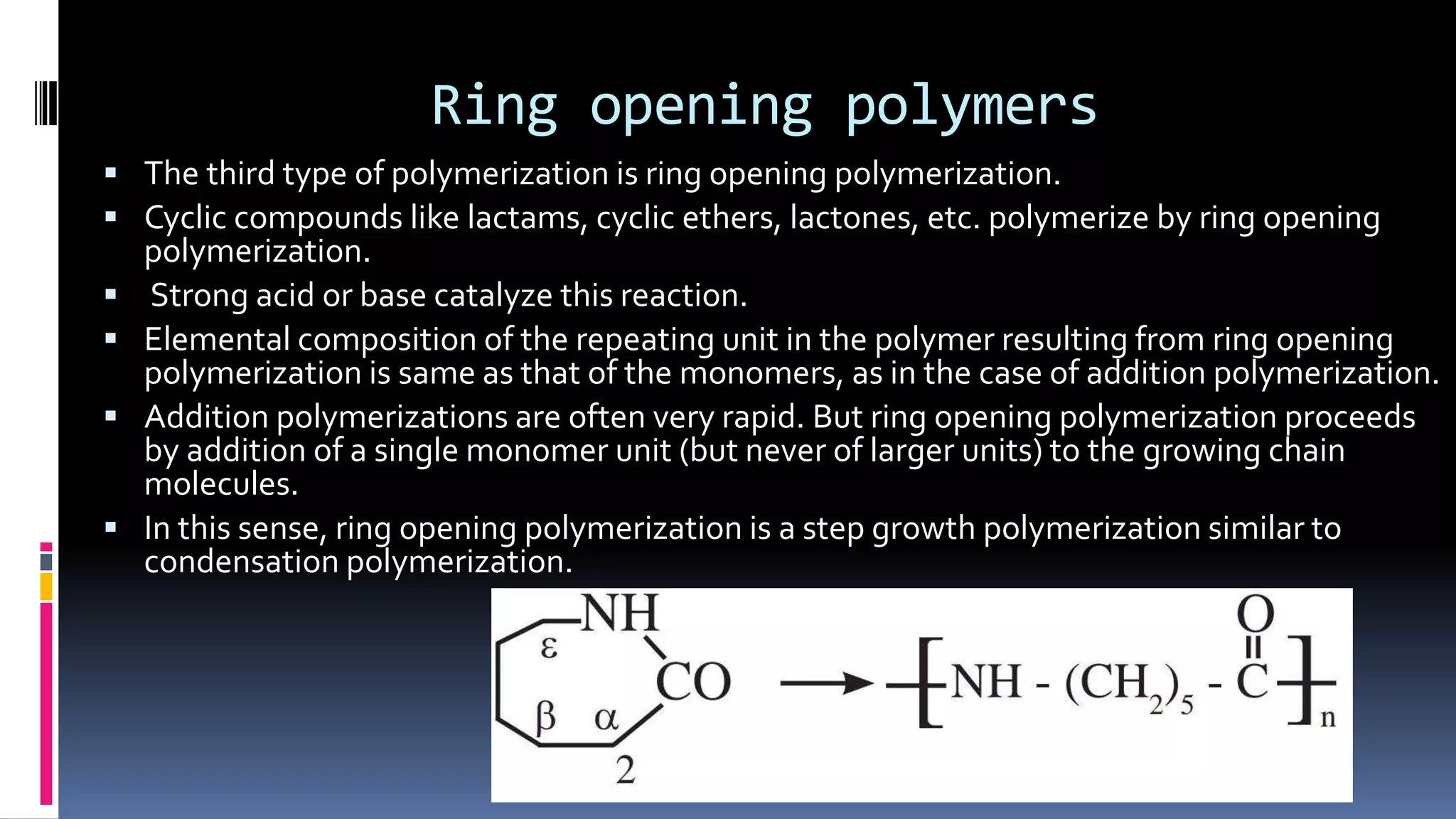
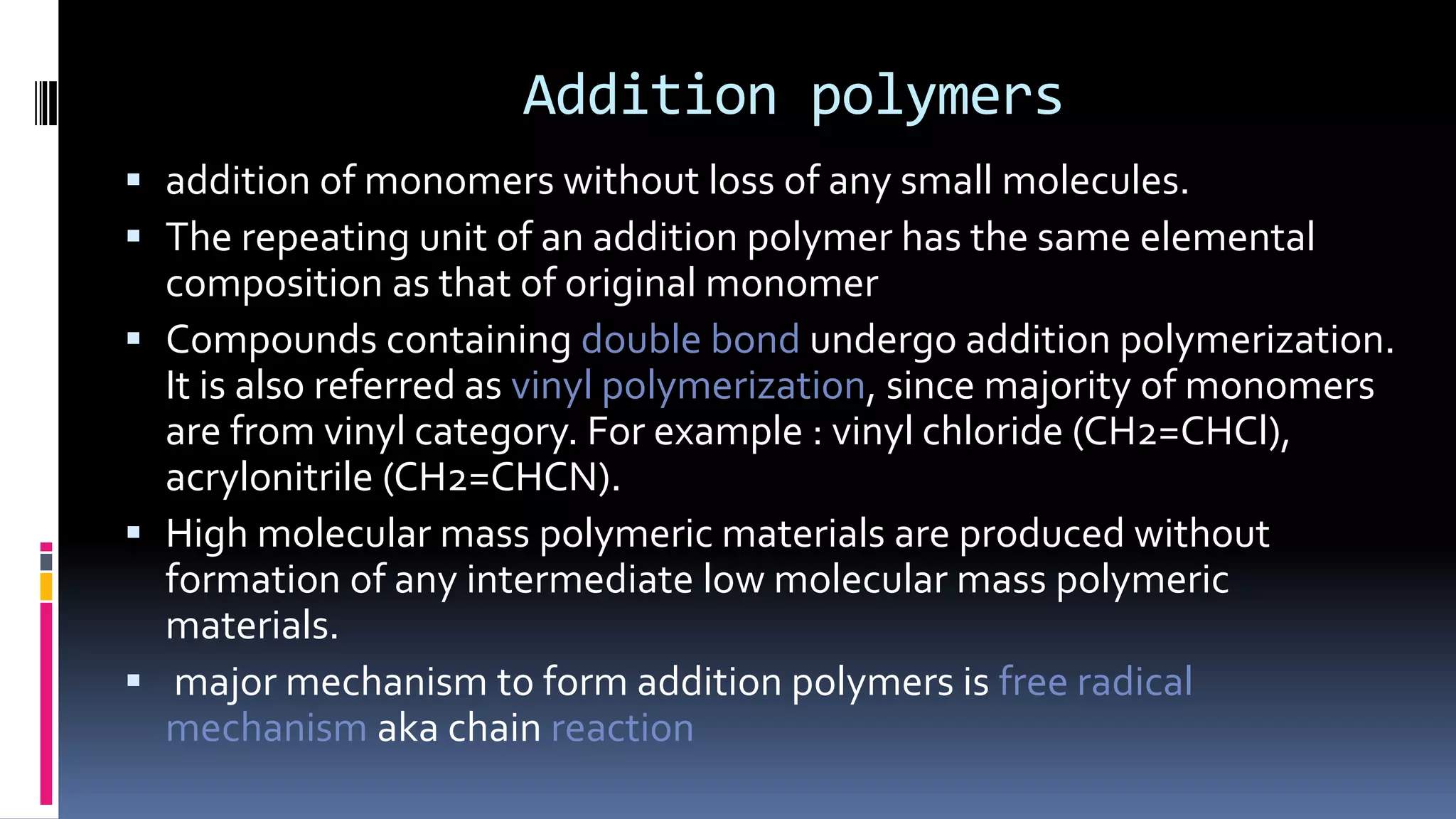
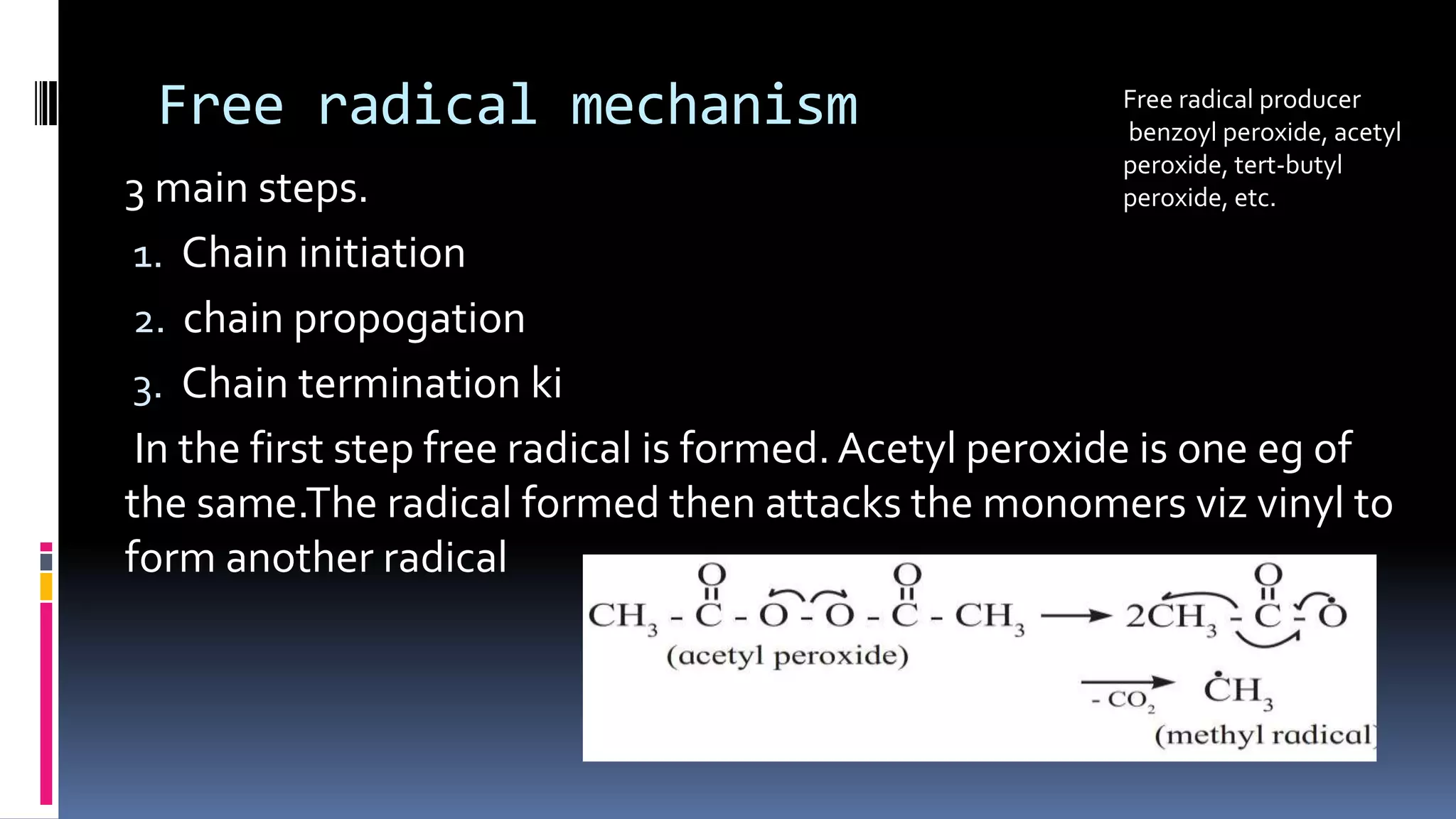
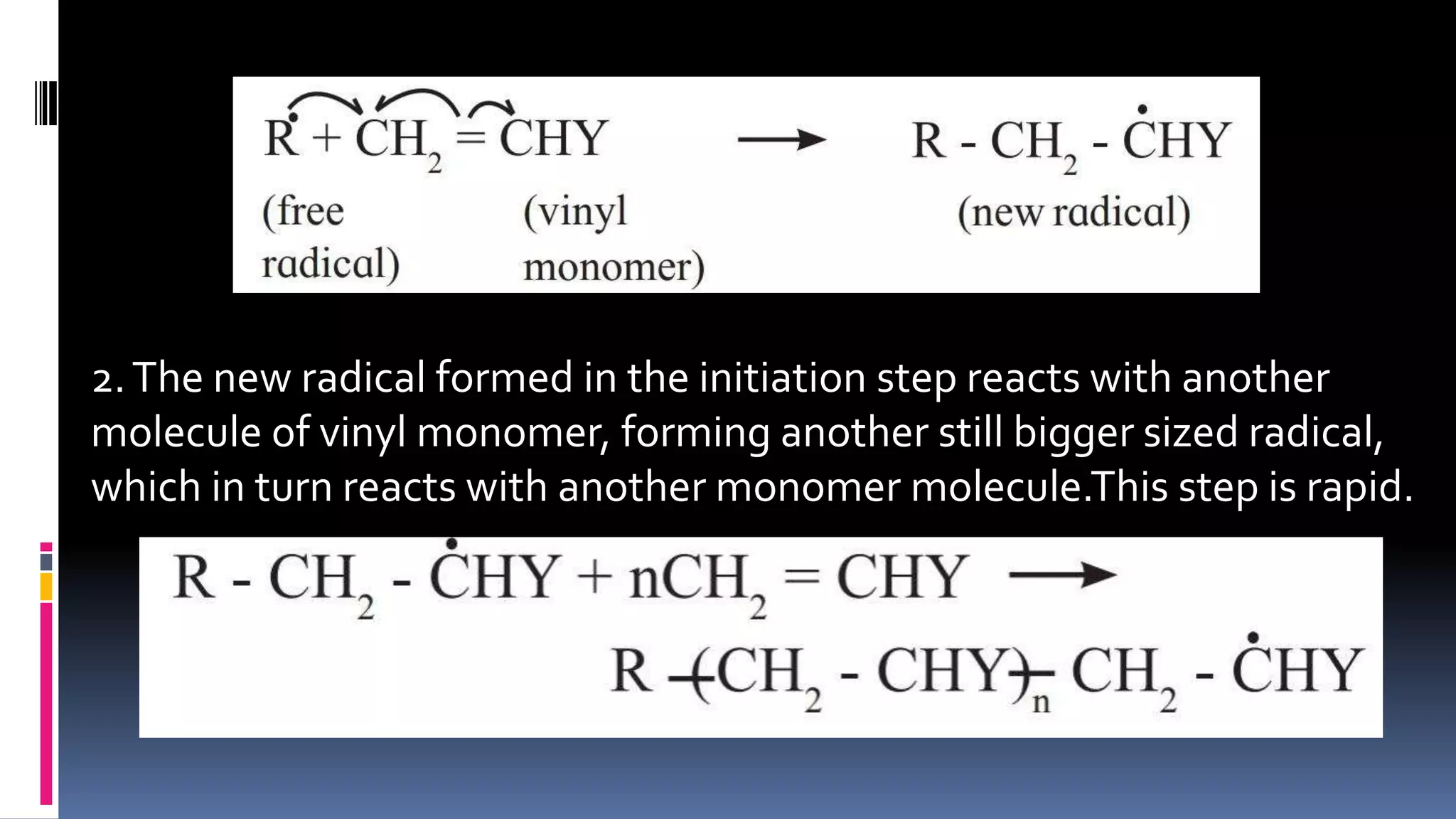
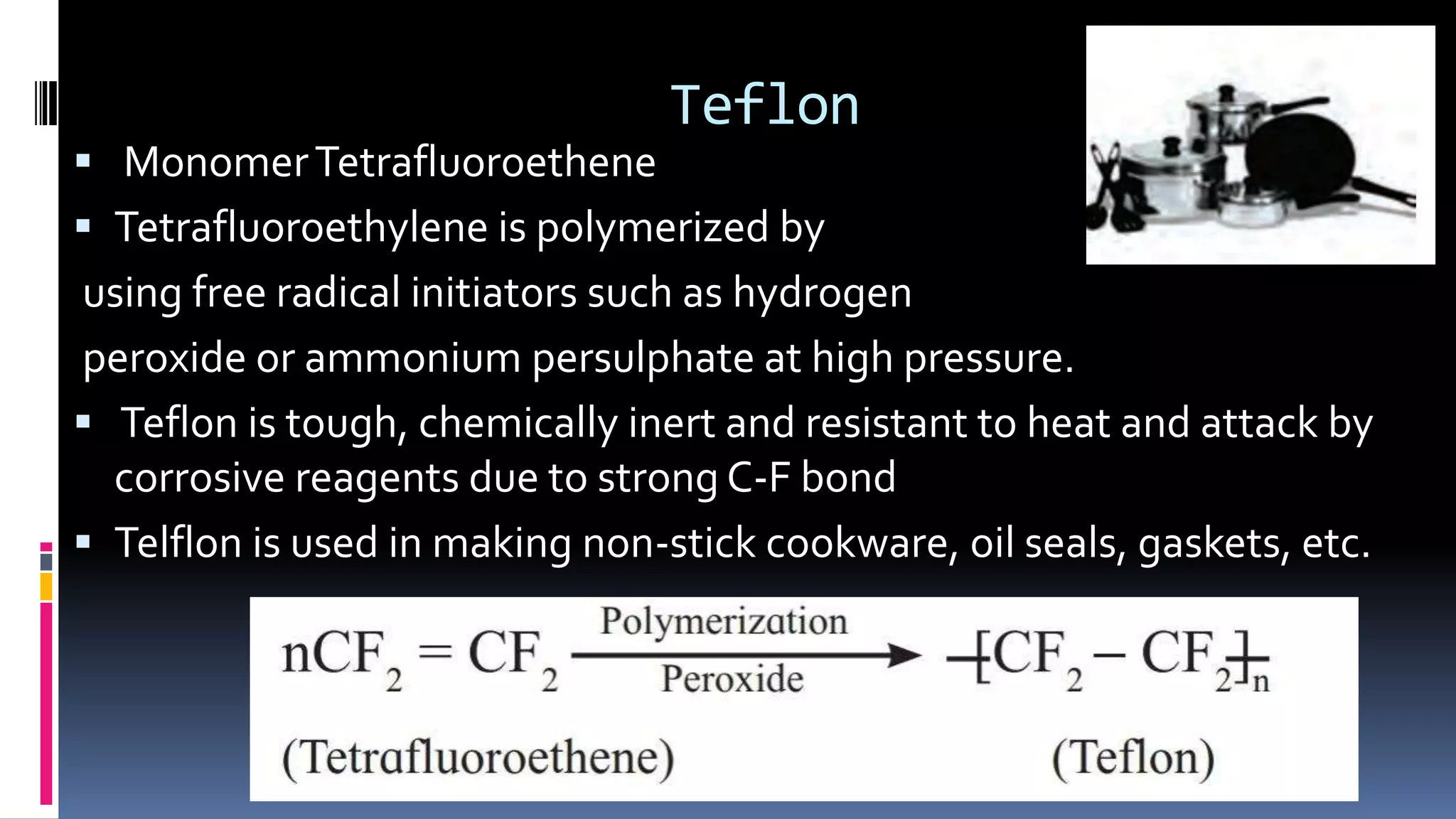
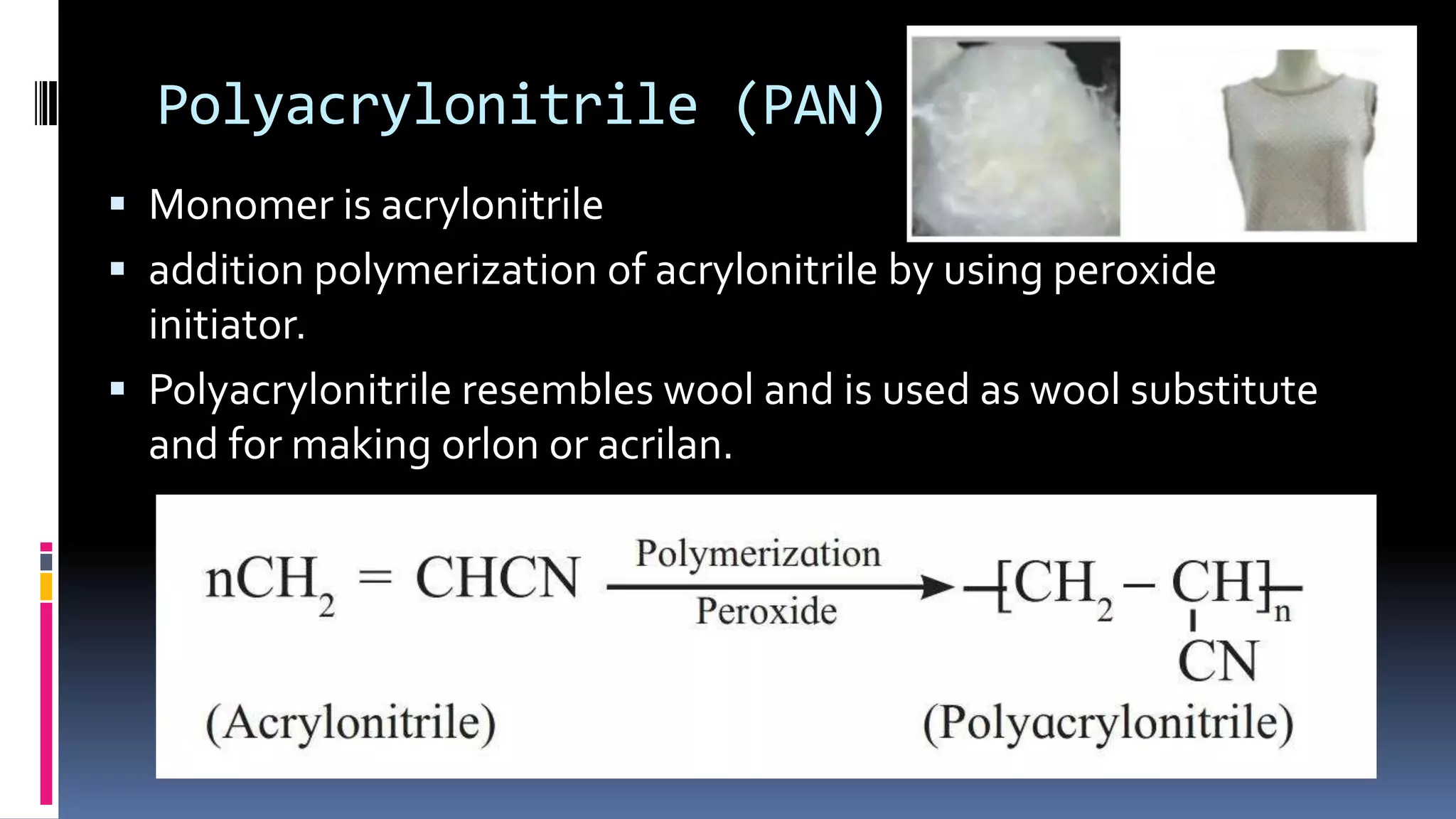
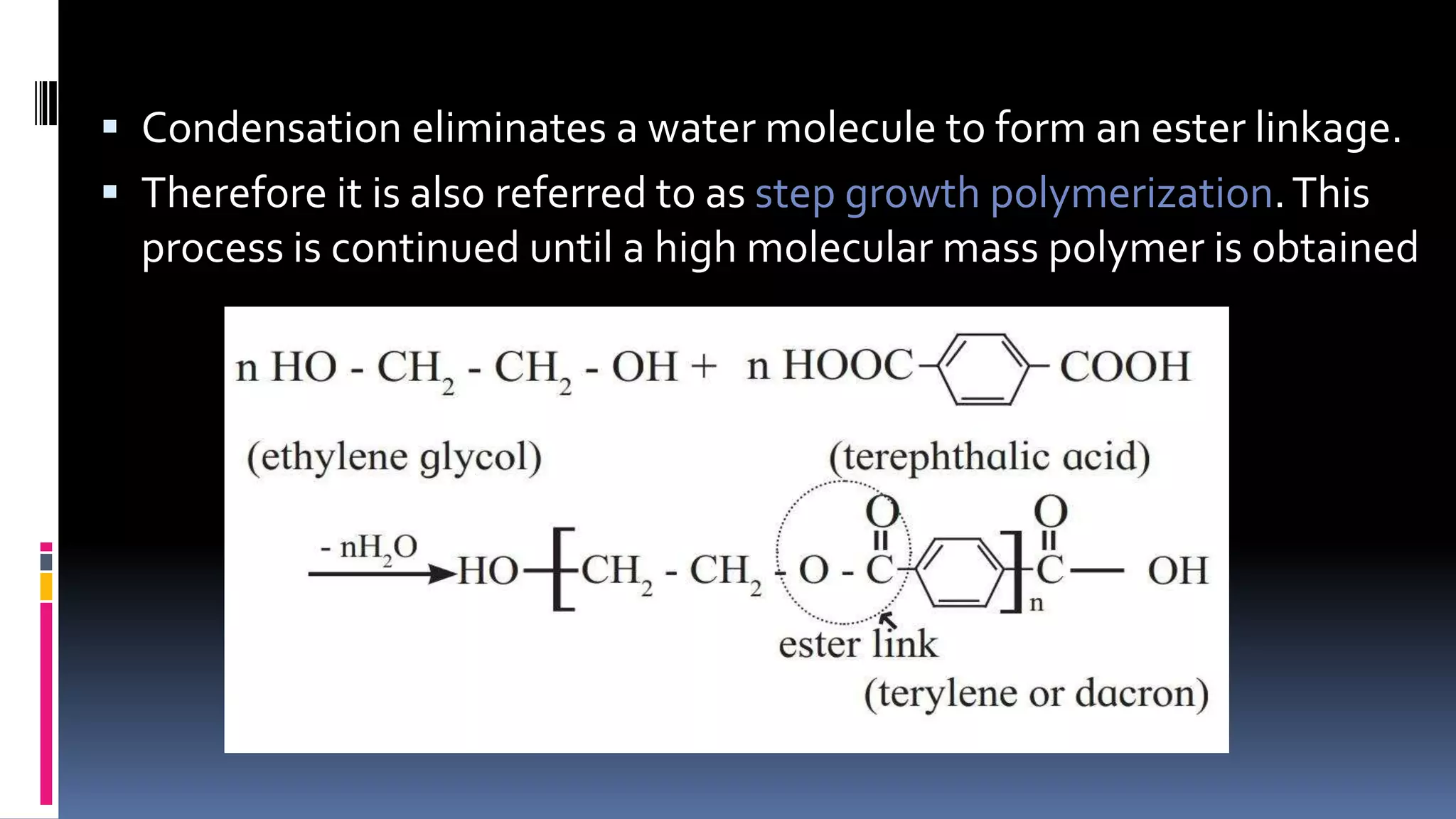
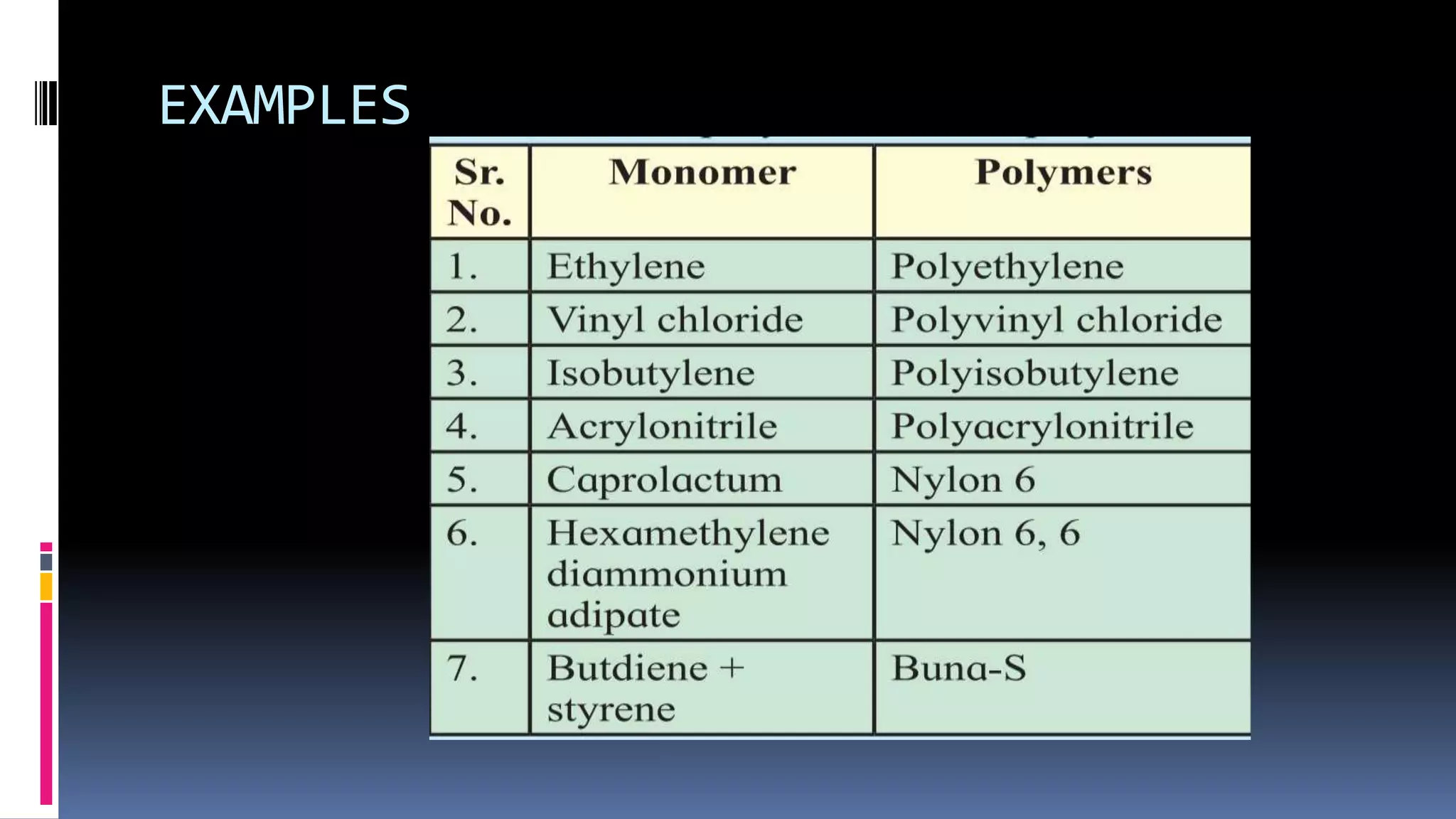
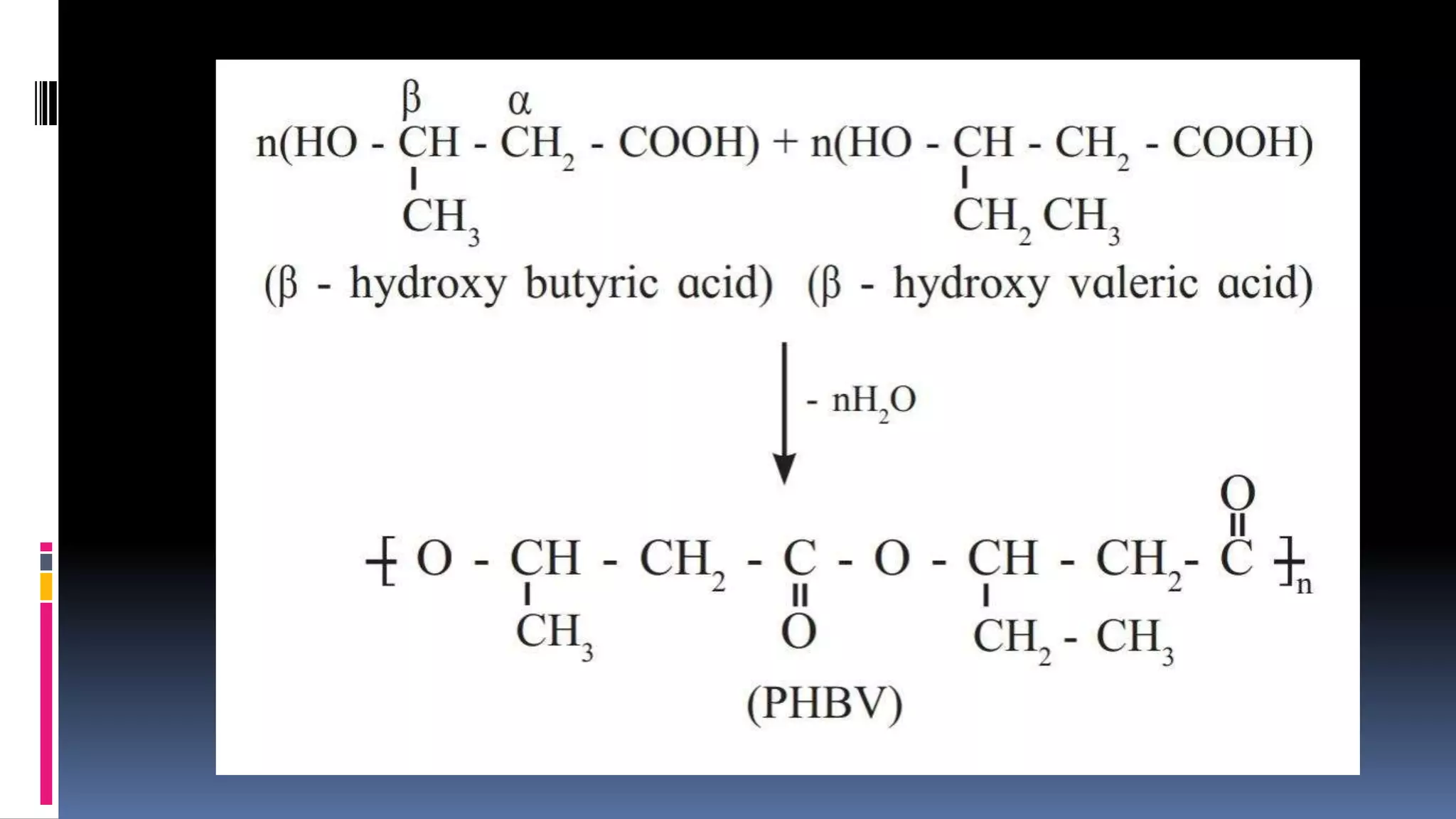
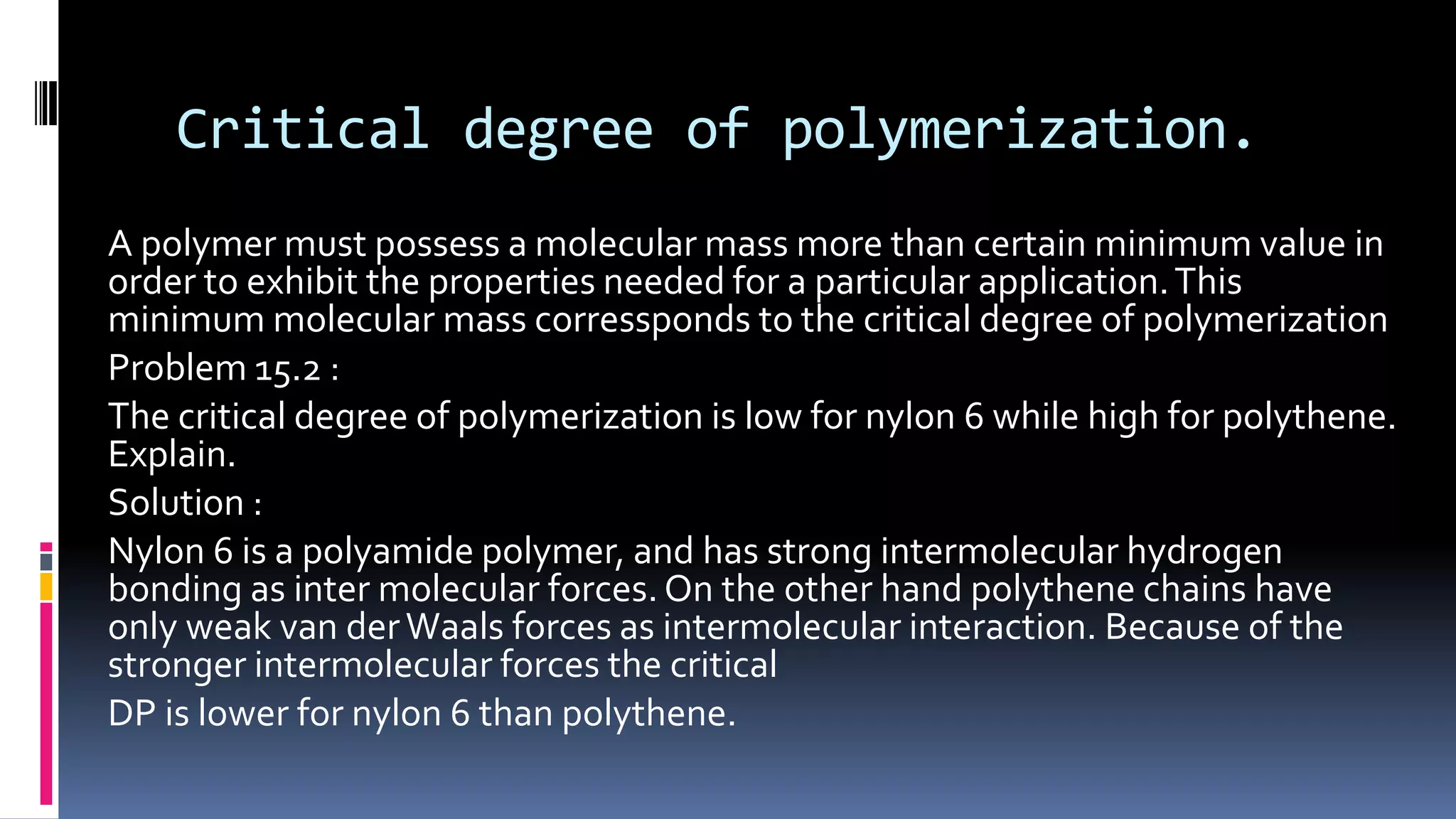
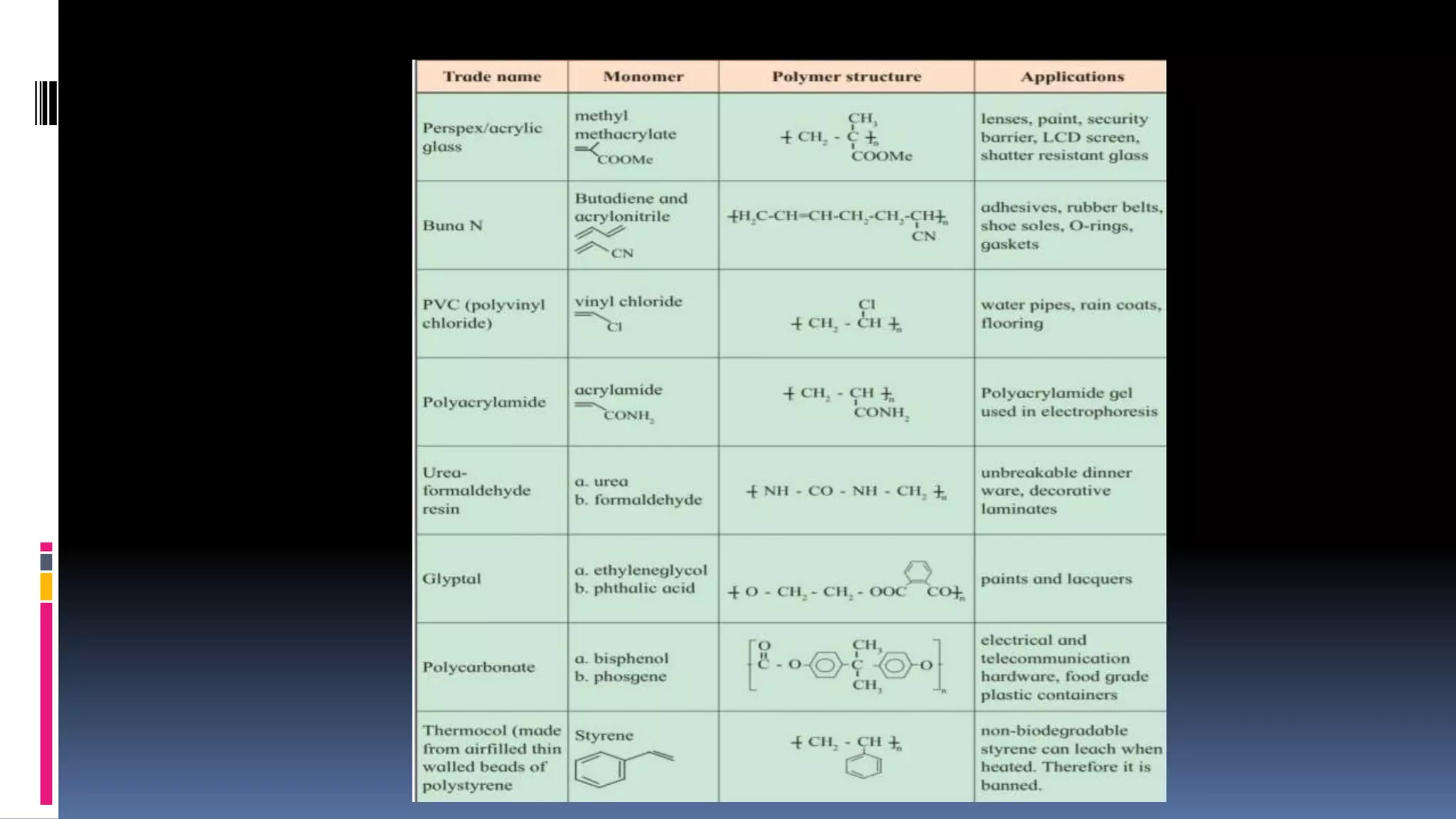
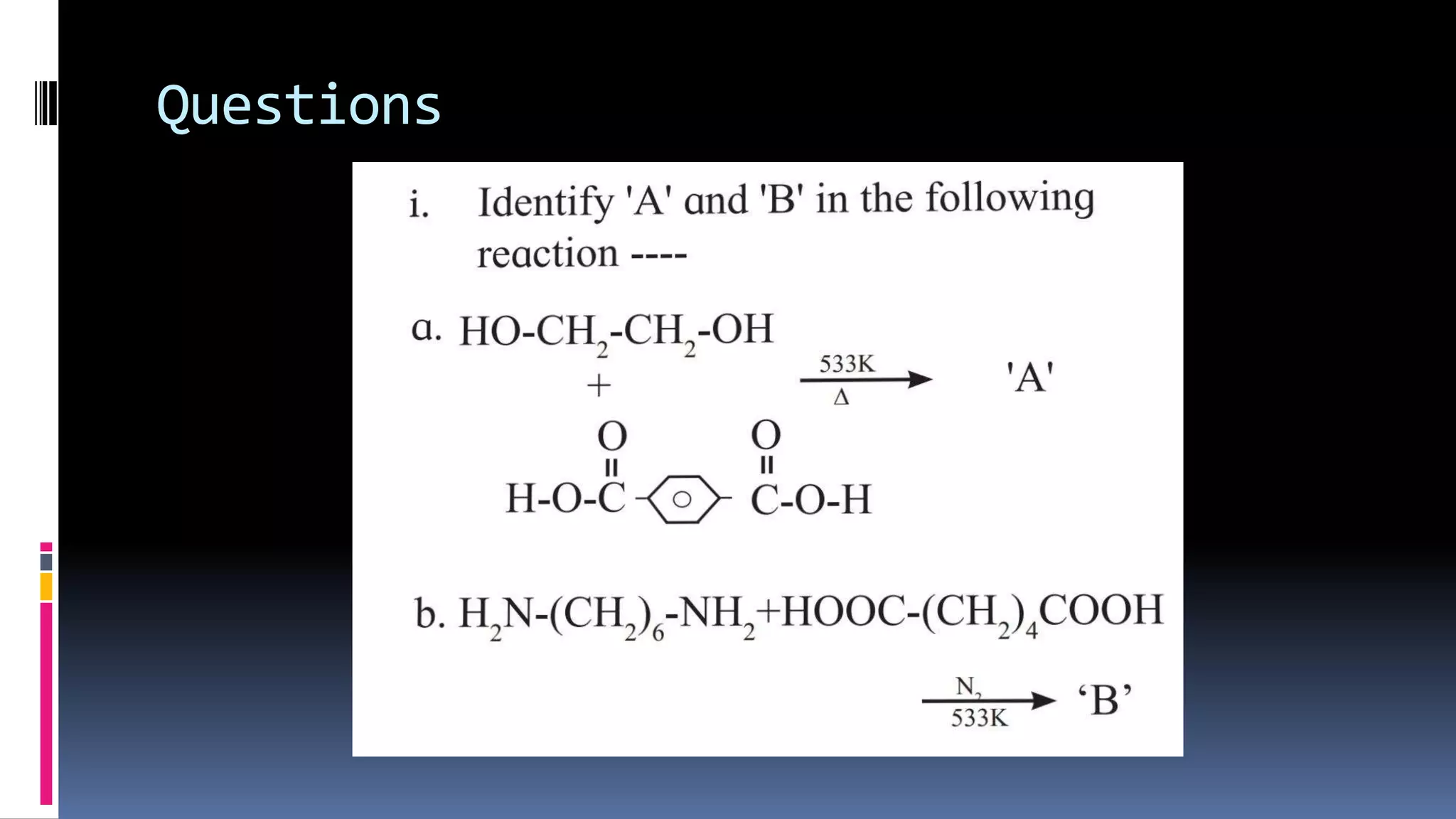
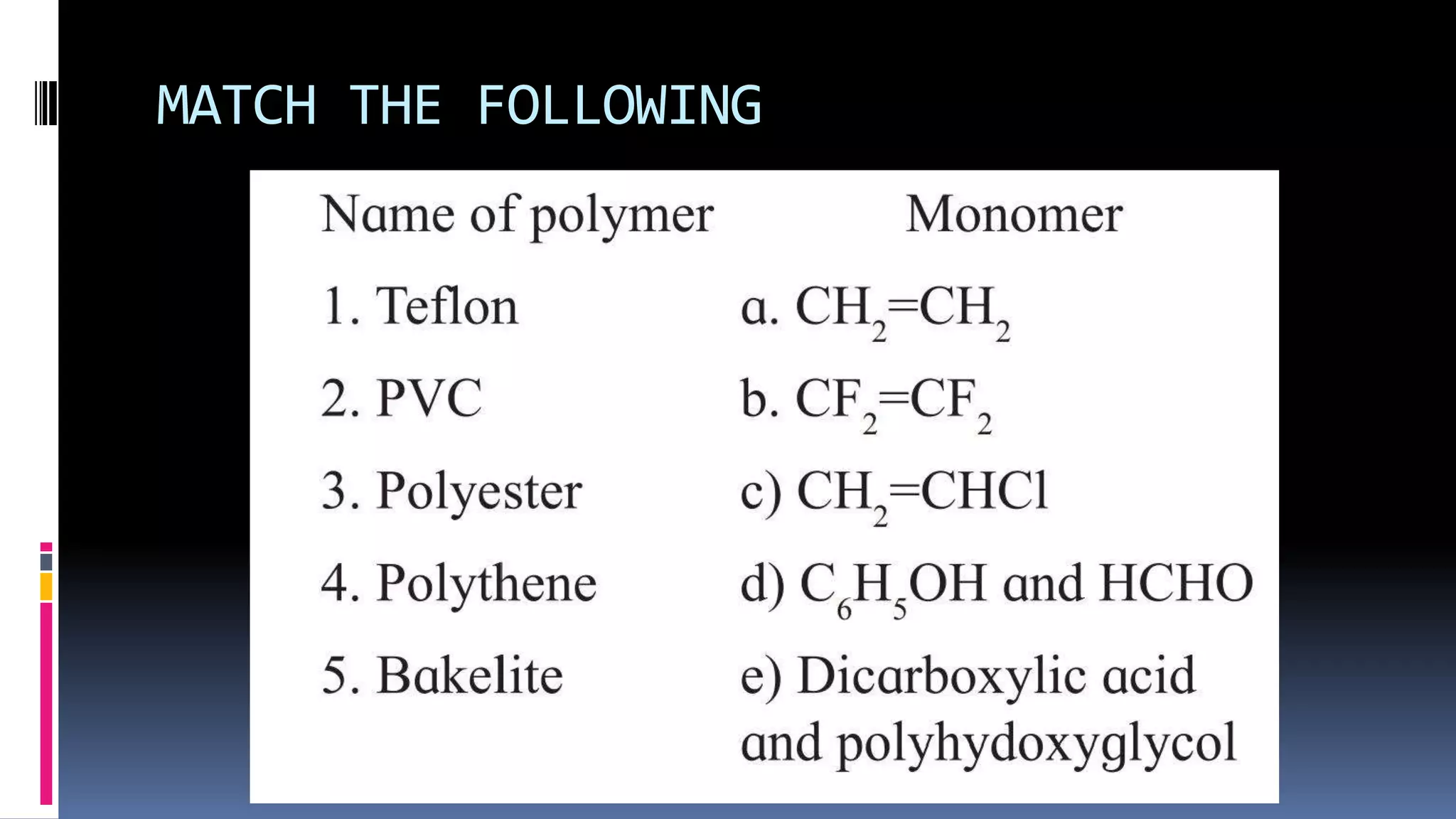
1. Polymers are large macromolecules formed by chemical bonding of repeating structural units called monomers.
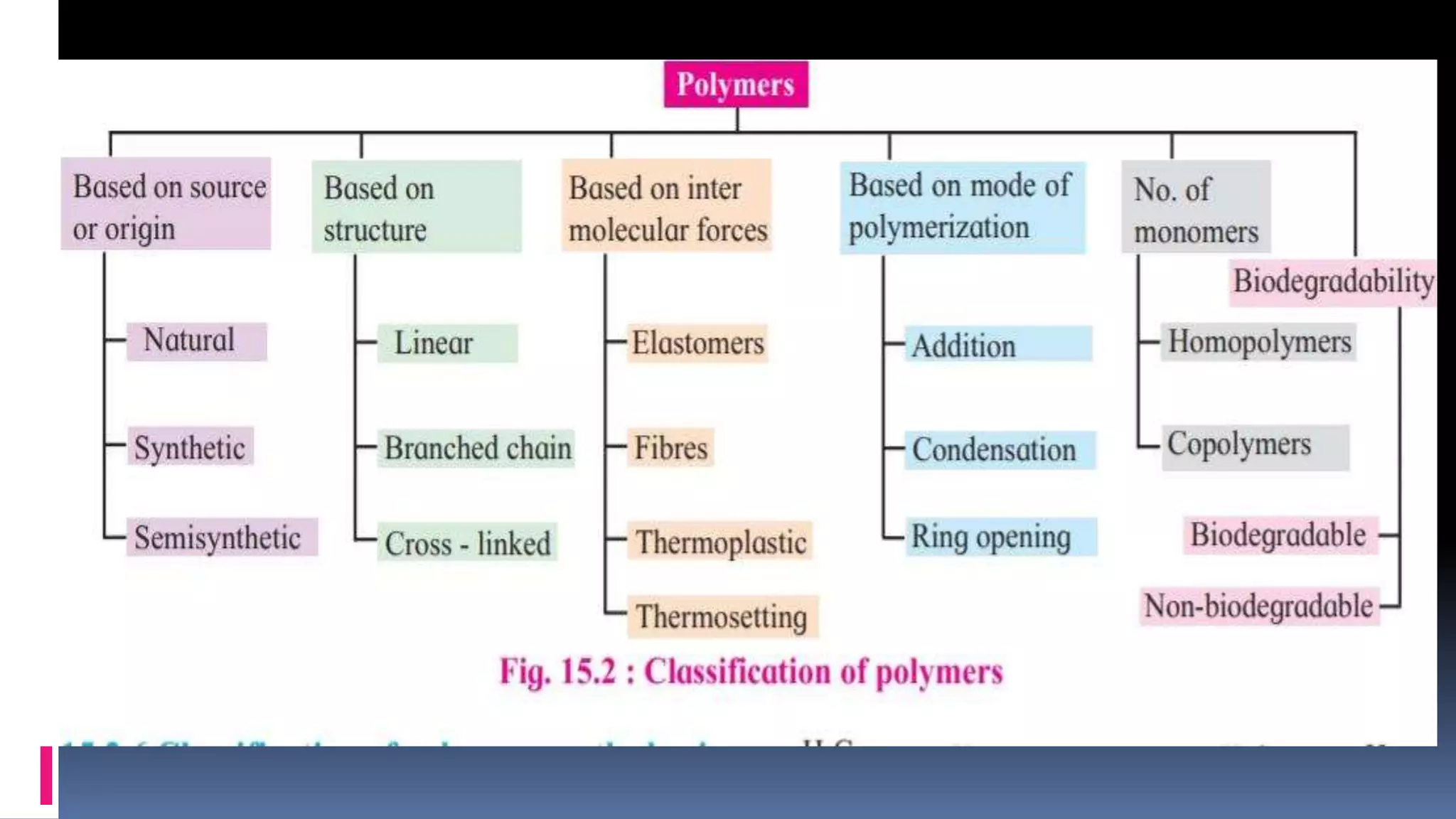
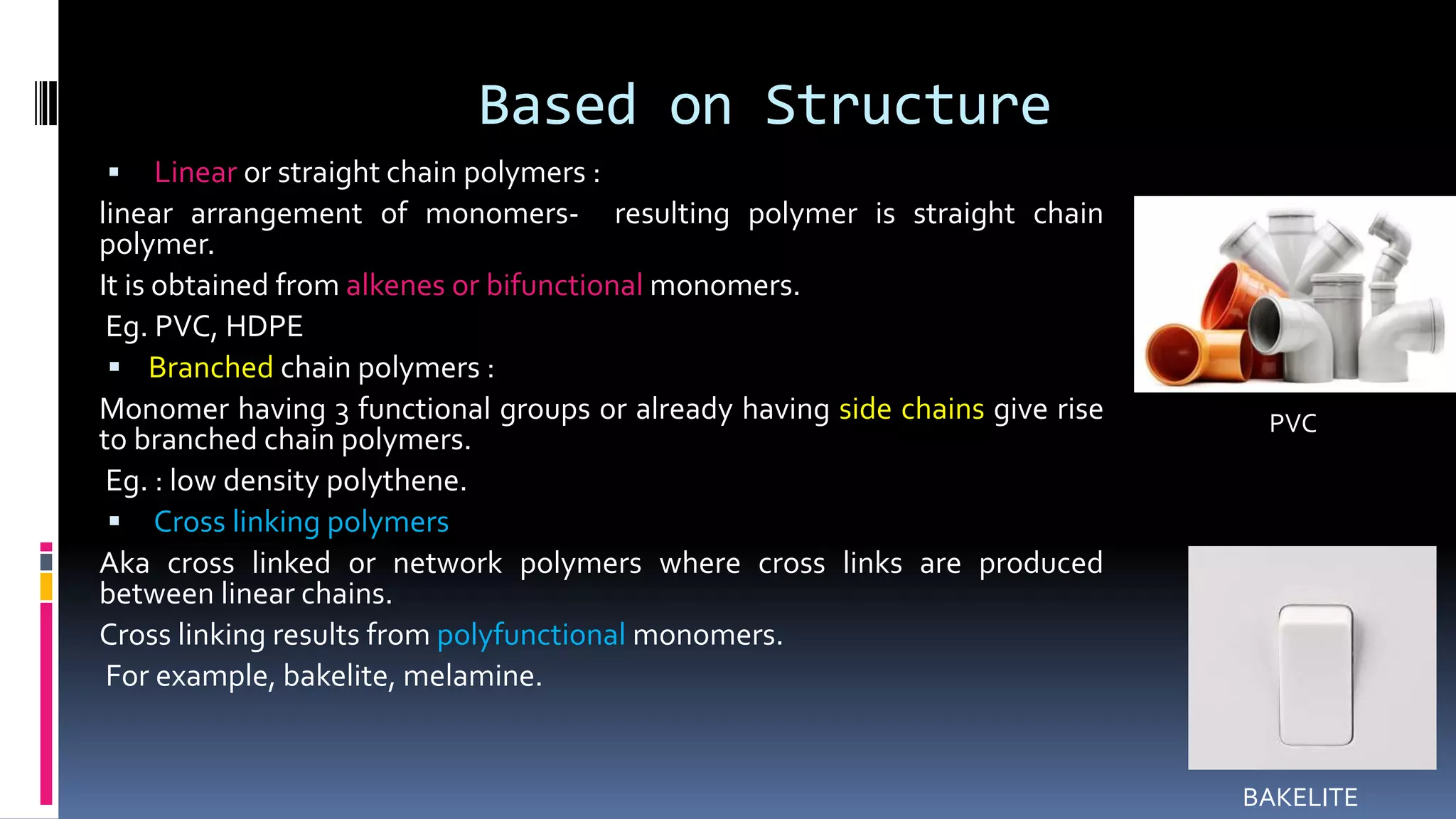
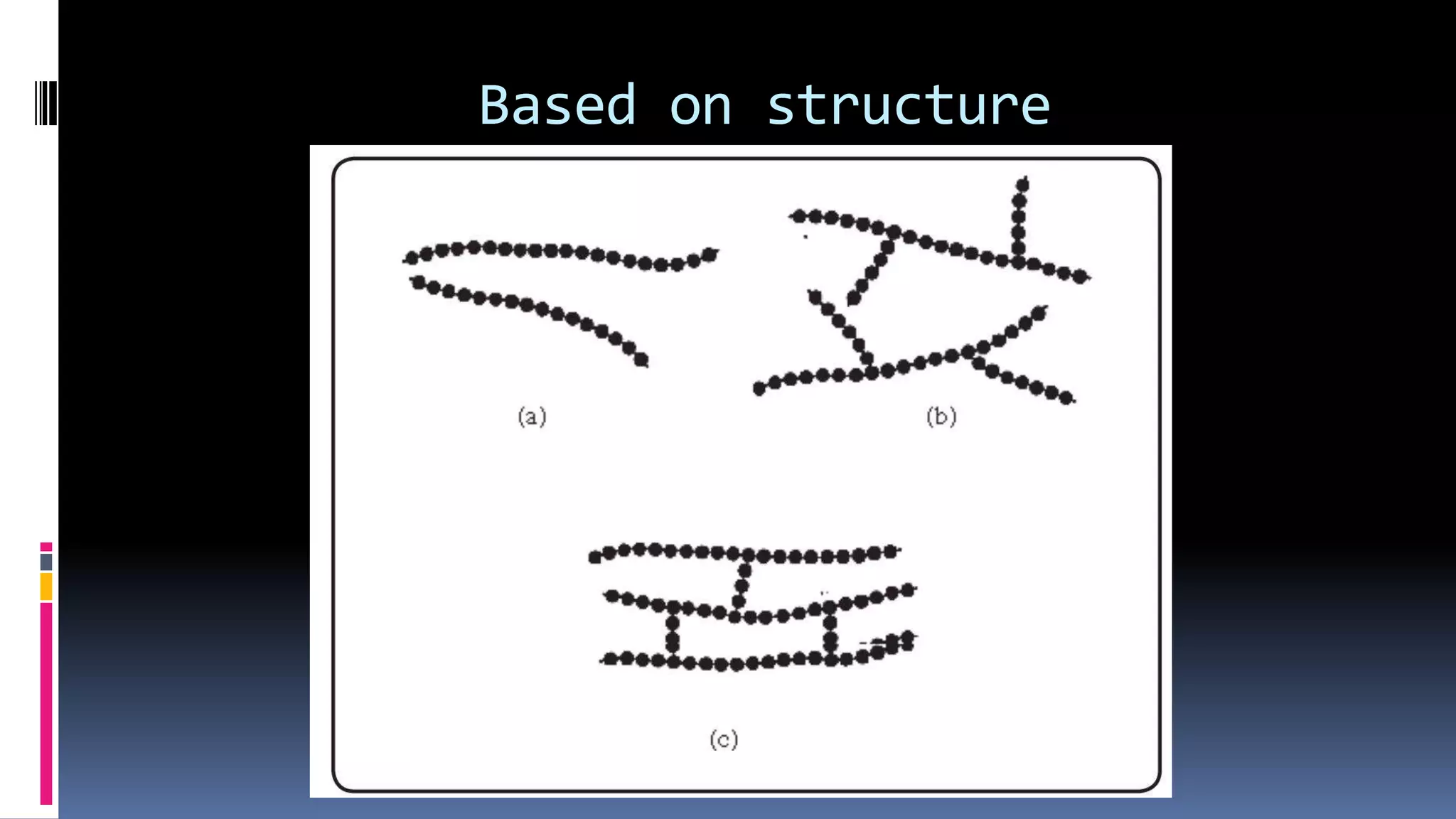
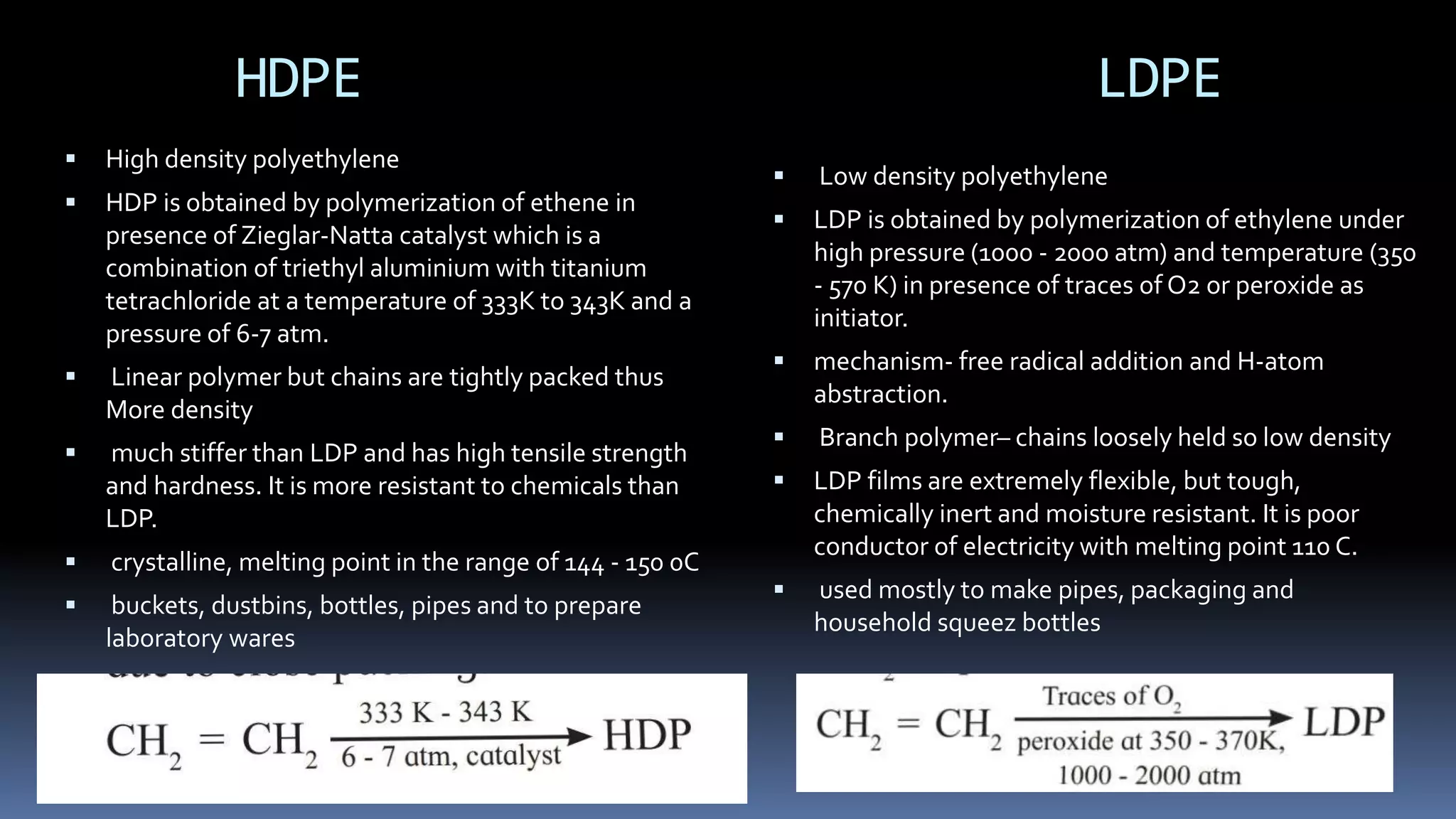
2. Polymers can be classified based on their source, structure, intermolecular forces, process of polymerization, types of monomers, and biodegradability.
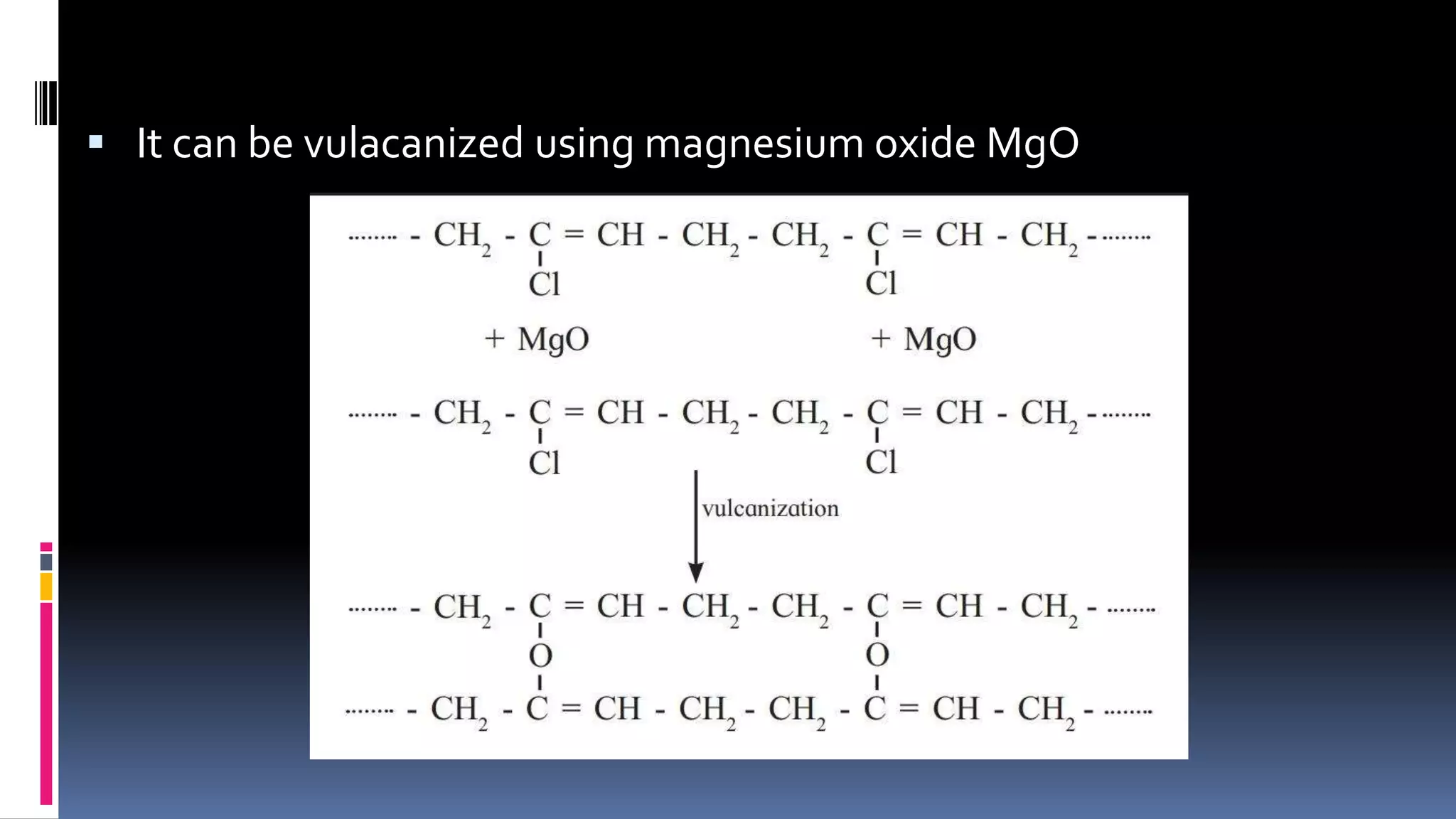
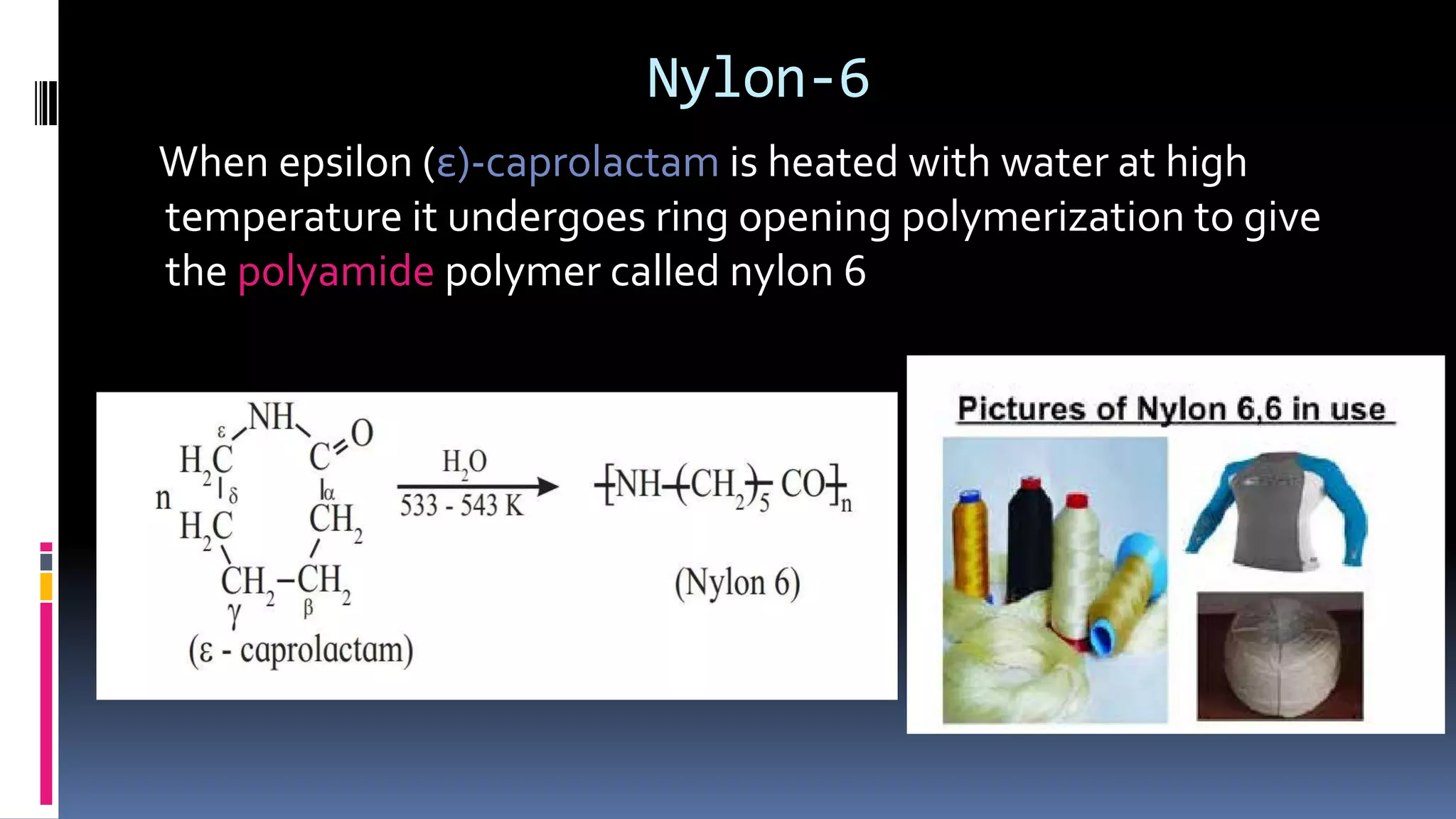
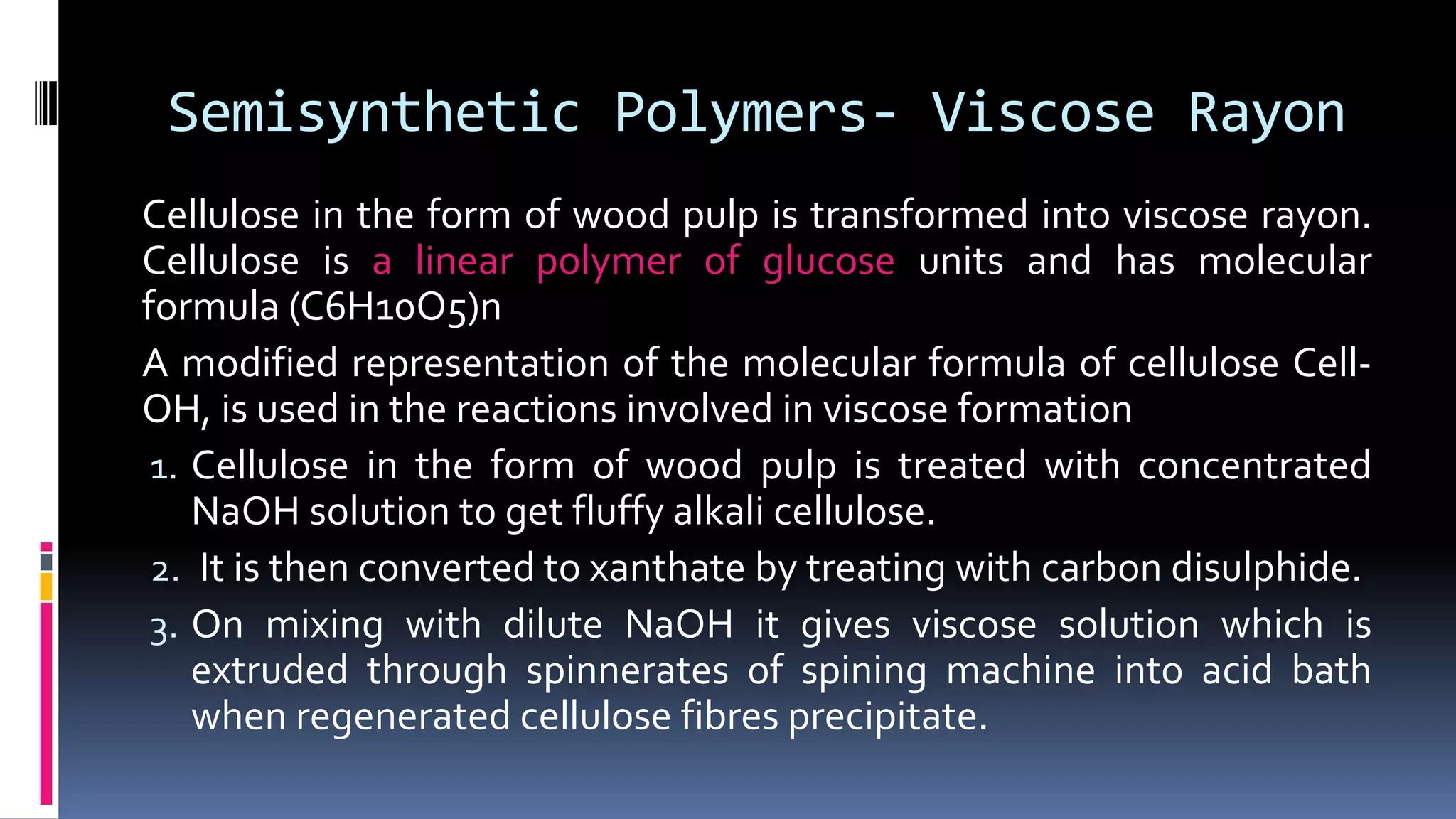
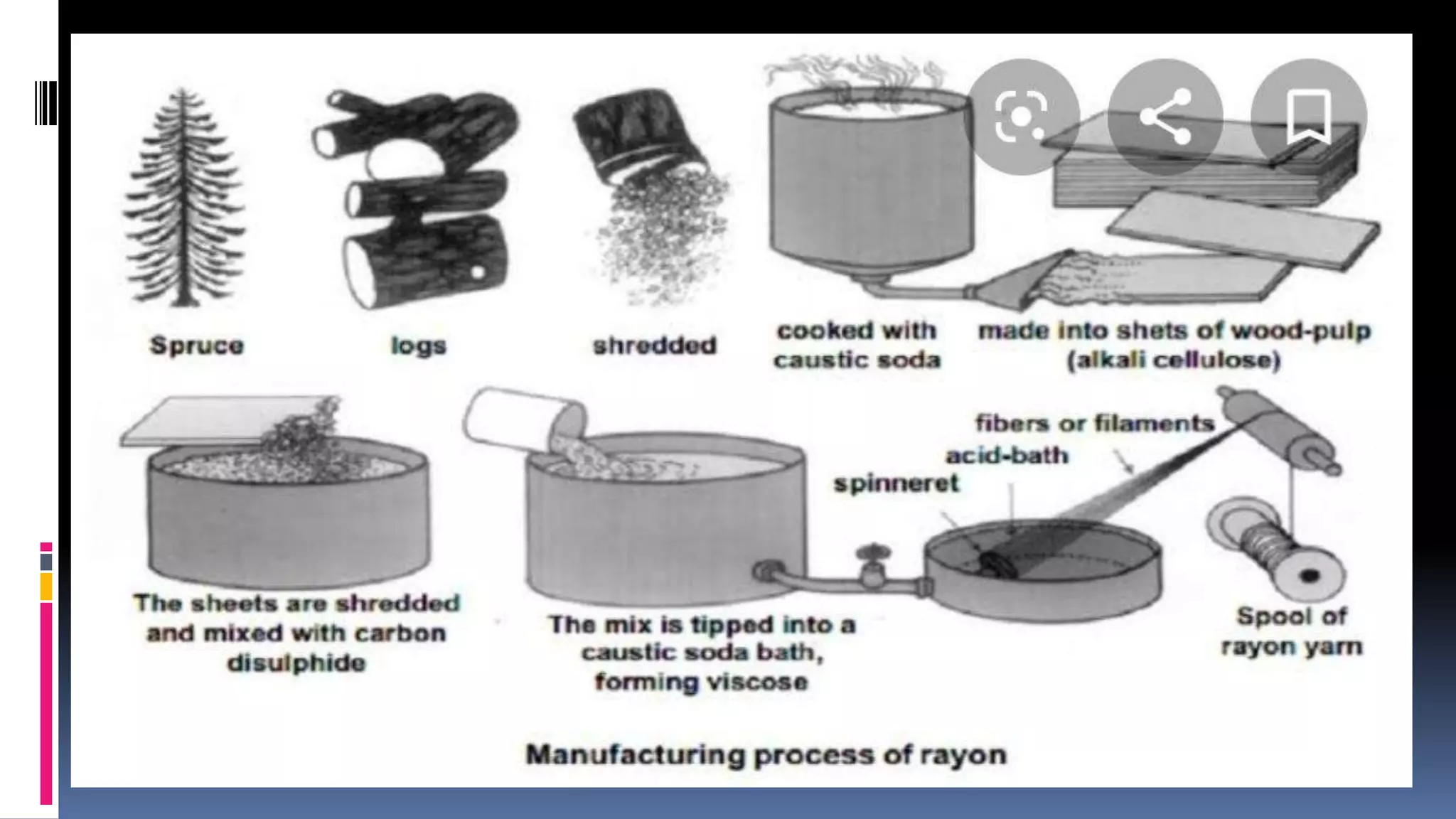
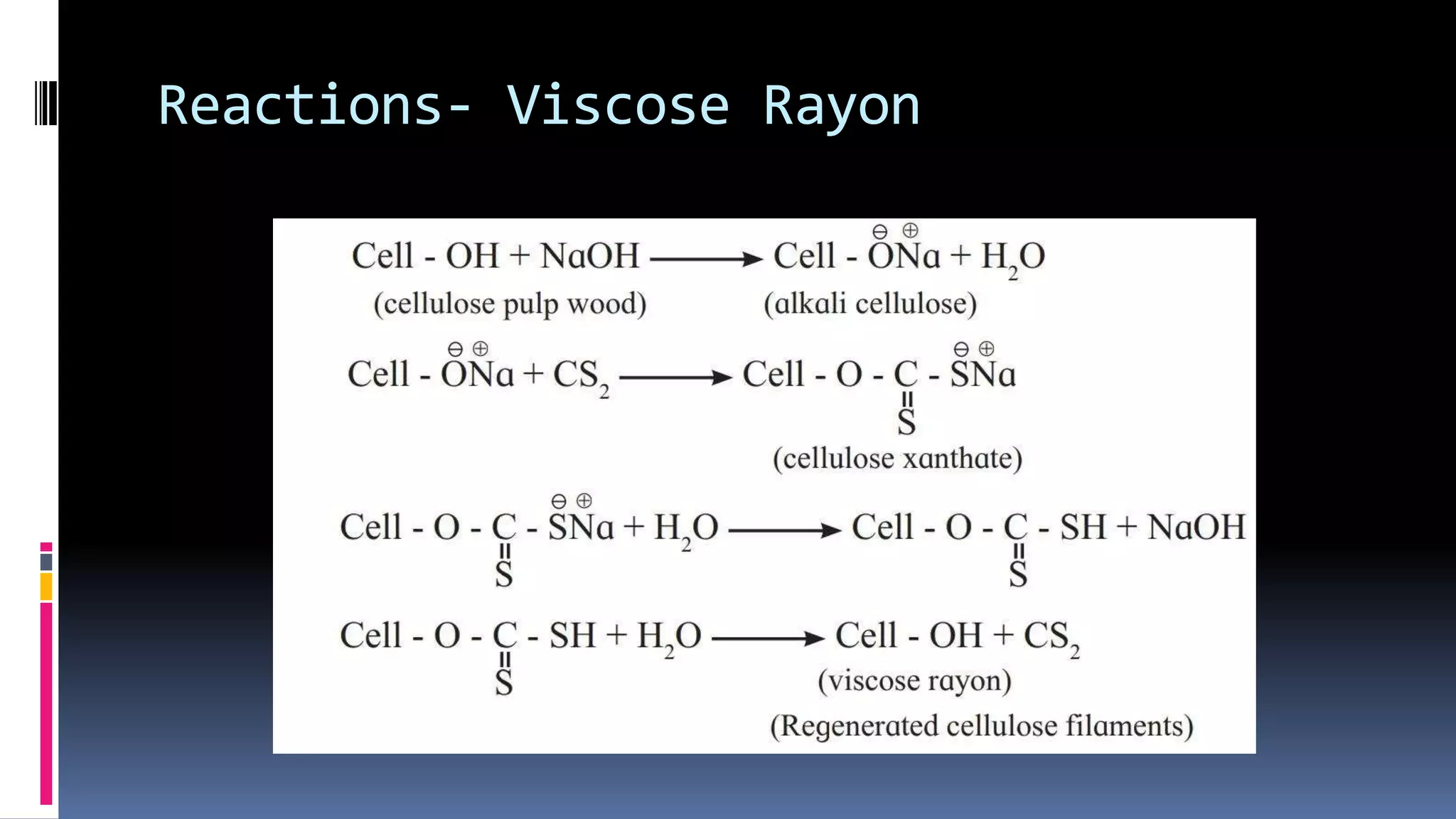
3. Common natural polymers include rubber from plants and silk/wool from animals, while synthetic polymers are man-made like nylon, polyester, and neoprene. Semisynthetic polymers are derived from natural polymers like rayon.