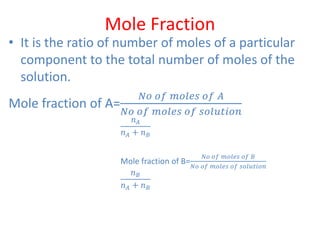
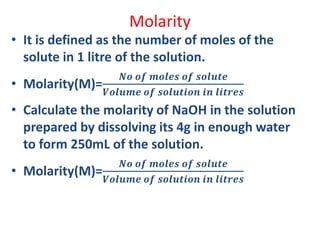
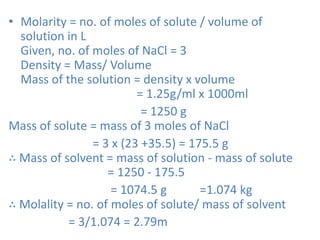
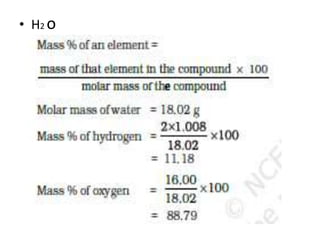
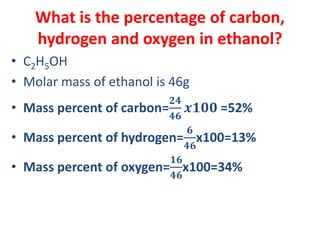
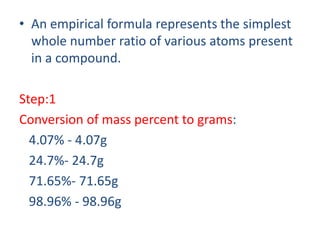
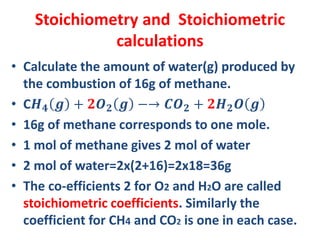
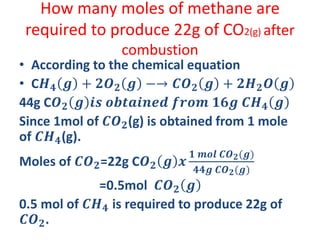
The document covers fundamental concepts of chemistry for Class 11, including the importance, applications, and calculations related to atoms, molecules, and stoichiometry. It highlights chemistry's role in agriculture, health, and environmental protection, along with detailed explanations of atomic and molecular masses, the mole concept, and practical applications in industry. Key topics such as percentage composition, empirical and molecular formulas, as well as stoichiometric calculations, are also addressed, providing essential knowledge for students.

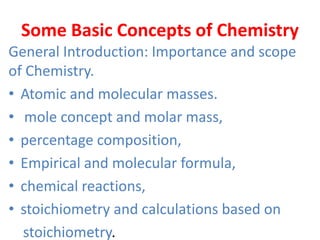









![• N2+3H2⟶2NH3
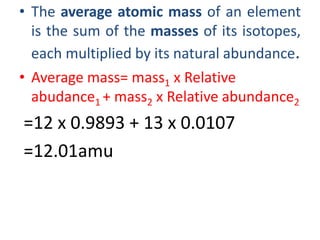
• Molecular mass of Nitrogen =28g/mol=0.028kg/mol
• Molecular mass of Hydrogen =6g/mol=0.006kg/mol
• Molecular mass of Ammonia =17g/mol=0.017kg/mol
• Now, according to the balanced chemical equation,
• 0.028 kg of Nitrogen reacts with 0.006 kg of Hydrogen.
∴ 50 kg of Nitrogen reacts with[
(𝟎.𝟎𝟎𝟔𝒙𝟓𝟎)
𝟎.𝟎𝟐𝟖
] =10.71kg of
Hydrogen.
• The amount of Hydrogen (given 10 kg) is less than
the amount required (i.e., 10.71 kg) for 50 kg of
Nitrogen.
• Therefore, Hydrogen is the limiting reagent.](https://image.slidesharecdn.com/somebasicconcepts-copy-201129084923/85/Some-basic-concepts-copy-25-320.jpg)