Bohrsmodelforhydrogenatom 141216074739-conversion-gate02
•Download as PPT, PDF•
0 likes•376 views
Its use for every students for this ppt on Bohr"s Postulates.
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Atom, origin of spectra Bohr's theory of hydrogen atom

Atom, origin of spectra Bohr's theory of hydrogen atom
How the Bohr Model of the Atom Accounts for Limitations with Classical Mechan...

How the Bohr Model of the Atom Accounts for Limitations with Classical Mechan...
Chemistry - Chp 5 - Electrons In Atoms - Powerpoint

Chemistry - Chp 5 - Electrons In Atoms - Powerpoint
Viewers also liked
Viewers also liked (20)
Similar to Bohrsmodelforhydrogenatom 141216074739-conversion-gate02
Similar to Bohrsmodelforhydrogenatom 141216074739-conversion-gate02 (20)
202006151236284892NK-Bohr Model of Hydrogen Atom.pdf

202006151236284892NK-Bohr Model of Hydrogen Atom.pdf
enc=encoded=PWW_dKfjbHrN9xq3SPtoL41DH0Bw5FrP4bCUo7yCo9hDDPhsJJZA_EXSSes=.pptx

enc=encoded=PWW_dKfjbHrN9xq3SPtoL41DH0Bw5FrP4bCUo7yCo9hDDPhsJJZA_EXSSes=.pptx
Compare and contrast the ideas that led to the Bohr model of the ato.pdf

Compare and contrast the ideas that led to the Bohr model of the ato.pdf
ELECTRONIC STRUCTURE OF ATOMS AND PERIODICITY (STU).pptx

ELECTRONIC STRUCTURE OF ATOMS AND PERIODICITY (STU).pptx
Recently uploaded
Call Girl Aurangabad Indira Call Now: 8617697112 Aurangabad Escorts Booking Contact Details WhatsApp Chat: +91-8617697112 Aurangabad Escort Service includes providing maximum physical satisfaction to their clients as well as engaging conversation that keeps your time enjoyable and entertaining. Plus they look fabulously elegant; making an impressionable. Independent Escorts Aurangabad understands the value of confidentiality and discretion - they will go the extra mile to meet your needs. Simply contact them via text messaging or through their online profiles; they'd be more than delighted to accommodate any request or arrange a romantic date or fun-filled night together. We provide –(INDIRA) Call Girl Aurangabad Call Now 8617697112 Aurangabad Escorts 24x7

(INDIRA) Call Girl Aurangabad Call Now 8617697112 Aurangabad Escorts 24x7Call Girls in Nagpur High Profile Call Girls
Process of Integration the Laser Scan Data into FEA Model and Level 3 Fitness-for-Service Assessment of Critical Assets in Refinery & Process IndustriesFEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads

FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced LoadsArindam Chakraborty, Ph.D., P.E. (CA, TX)
Call girls in delhi ✔️✔️🔝 9953056974 🔝✔️✔️Welcome To Vip Escort Services In Delhi [ ]Noida Gurgaon 24/7 Open Sex Escort Services With Happy Ending ServiCe Done By Most Attractive Charming Soft Spoken Bold Beautiful Full Cooperative Independent Escort Girls ServiCe In All-Star Hotel And Home Service In All Over Delhi, Noida, Gurgaon, Faridabad, Ghaziabad, Greater Noida,
• IN CALL AND OUT CALL SERVICE IN DELHI NCR
• 3* 5* 7* HOTELS SERVICE IN DELHI NCR
• 24 HOURS AVAILABLE IN DELHI NCR
• INDIAN, RUSSIAN, PUNJABI, KASHMIRI ESCORTS
• REAL MODELS, COLLEGE GIRLS, HOUSE WIFE, ALSO AVAILABLE
• SHORT TIME AND FULL TIME SERVICE AVAILABLE
• HYGIENIC FULL AC NEAT AND CLEAN ROOMS AVAIL. IN HOTEL 24 HOURS
• DAILY NEW ESCORTS STAFF AVAILABLE
• MINIMUM TO MAXIMUM RANGE AVAILABLE.
Call Girls in Delhi & Independent Escort Service –
CALL GIRLS SERVICE DELHI NCR
Vip call girls in Delhi
Call Girls in Delhi, Call Girl Service 24×7 open
Call Girls in Delhi Best Delhi Escorts in Delhi
Low Rate Call Girls In Saket Delhi
X~CALL GIRLS IN Ramesh Nagar Metro
best Delhi call girls and Delhi escort service.
CALL GIRLS SERVICE IN ALL DELHI …
(Delhi) Call Girls in (Chanakyapuri)
Hot And Sexy Independent Model Escort Service In Delhi Unlimited Enjoy Genuine 100% Profiles And Trusted Door Step Call Girls Feel Free To Call Us Female Service Hot Busty & Sexy Party Girls Available For Complete Enjoyment. We Guarantee Full Satisfaction & In Case Of Any Unhappy Experience, We Would Refund Your Fees, Without Any Questions Asked. Feel Free To Call Us Female Service Provider Hours Opens Thanks.
Delhi Escorts Services 100% secure Services.Incall_OutCall Available and outcall Services provide.
We are available 24*7 for Full Night and short Time Escort Services all over Delhi NCR.
Delhi All Hotel Services available 3* 4* 5* Call Call
Delhi Escorts Services And Delhi Call Girl Agency 100% secure Services in my agency. Incall and outcall Services provide.
We are available 24*7 for Full Night and short Time Escort Services my agency in all over New Delhi
Delhi All Hotel Services available my agency
SERVICES [✓✓✓]
Housewife
College Girl
VIP Escort
Independent Girl
Aunty
Without a Condom sucking )?
Sexy Aunty.DSL (Dick Sucking Lips)?
DT (Dining at the Toes English Spanking)
Doggie (Sex style from no behind)??
OutCall- All Over Delhi Noida Gurgaon 24/7
FOR APPOINTMENT Call/Whatsop / 9953056974Call Girls in Netaji Nagar, Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service

Call Girls in Netaji Nagar, Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service9953056974 Low Rate Call Girls In Saket, Delhi NCR
From customer value engagements to hands-on production support, our Services span across every stage of our customers digital transformation journey, to help ensure that every customer is successful in their adoption of our solutions.
• Implementation, Upgrade, Migration, and Maintenance Services
• On-Premises and On-Cloud
• COTS Training Services; On-Site and Virtual
• Software Support Services; Legacy and 3DEXPERIENCE
• Value Engagement & Blueprinting
• Specialized Consulting and Support Services
• Customized Training Services
• Automation and Configuration Services
• Technical Resource Augmentation Services
• Project Management
• Know-how Training (mentoring) and Resource AugmentationNavigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...

Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...Arindam Chakraborty, Ph.D., P.E. (CA, TX)
Recently uploaded (20)
Top Rated Call Girls In chittoor 📱 {7001035870} VIP Escorts chittoor

Top Rated Call Girls In chittoor 📱 {7001035870} VIP Escorts chittoor
XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX

XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
FULL ENJOY Call Girls In Mahipalpur Delhi Contact Us 8377877756

FULL ENJOY Call Girls In Mahipalpur Delhi Contact Us 8377877756
Call Girls Pimpri Chinchwad Call Me 7737669865 Budget Friendly No Advance Boo...

Call Girls Pimpri Chinchwad Call Me 7737669865 Budget Friendly No Advance Boo...
VIP Model Call Girls Kothrud ( Pune ) Call ON 8005736733 Starting From 5K to ...

VIP Model Call Girls Kothrud ( Pune ) Call ON 8005736733 Starting From 5K to ...
Design For Accessibility: Getting it right from the start

Design For Accessibility: Getting it right from the start
(INDIRA) Call Girl Aurangabad Call Now 8617697112 Aurangabad Escorts 24x7

(INDIRA) Call Girl Aurangabad Call Now 8617697112 Aurangabad Escorts 24x7
FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads

FEA Based Level 3 Assessment of Deformed Tanks with Fluid Induced Loads
Block diagram reduction techniques in control systems.ppt

Block diagram reduction techniques in control systems.ppt
Call Girls in Netaji Nagar, Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service

Call Girls in Netaji Nagar, Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service
Standard vs Custom Battery Packs - Decoding the Power Play

Standard vs Custom Battery Packs - Decoding the Power Play
Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...

Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...
DC MACHINE-Motoring and generation, Armature circuit equation

DC MACHINE-Motoring and generation, Armature circuit equation
Bohrsmodelforhydrogenatom 141216074739-conversion-gate02
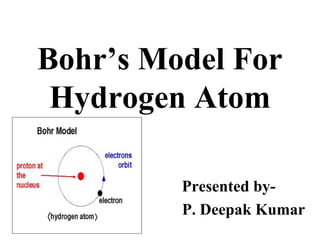
- 1. Bohr’s Model For Hydrogen Atom Presented by- P. Deepak Kumar
- 2. Overview of presentation • Bohr’s atomic model • Postulates of Bohr’s atomic model • Successes of Bohr’s model • Calculations based on Bohr’s model • Limitations of Bohr’s Model • Bibliography
- 4. Bohr’s Atomic Model • An atom is made up of three particles, electrons, protons and neutrons. Electrons have a negative charge and protons have a positive charge whereas neutrons have no charge. They are neutral. Due to the presence of equal number of negative electrons and positive protons, the atom as a whole is electrically neutral.
- 5. • The protons and electrons are located in a small nucleus at the centre of the atom. Due to the presence of protons, the nucleus is positively charged. • The electrons revolve rapidly around the nucleus in fixed circular paths called energy levels or shells. The 'energy levels' or 'shells' or 'orbits' are represented in two ways: either by the numbers 1, 2, 3, 4, 5 and 6 or by letters K, L, M, N, O and P. The energy levels are counted from centre outwards.
- 6. • Each energy level is associated with a fixed amount of energy. The shell nearest to the nucleus has minimum energy and the shell farthest from the nucleus has maximum energy. • There is no change in the energy of electrons as long as they keep revolving with the same energy level. But, when an electron jumps from a lower energy level to a higher one, some energy is absorbed while some energy is emitted.
- 7. • When an electron jumps from a higher energy level to a lower one, the amount of energy absorbed or emitted is given by the difference of energies associated with the two levels. Thus, if an electron jumps from orbit 1 (energy E1) to orbit 2 (energy E2), the change in energy is given by E2 - E1.
- 8. • The energy change is accompanied by absorption of radiation energy of E = E2 E1 = h where, h is a constant called 'Planck's constant' and is the frequency of radiation absorbed or emitted. The value of h is 6.626 x 10-34 J-s . The absorption and emission of light due to electron jumps are measured by use of spectrometers.
- 9. Postulates of Bohr’s Atomic Model • Electrons revolve round the nucleus with definite velocities in concentric circular orbits situated at definite distances from the nucleus. The energy of an electron in a certain orbit remains constant. As long as it remains in that orbit, it neither emits nor absorbs energy. These are termed stationary states or main energy states.
- 10. • Bohr proposed that the angular momentum of an electron is quantized. Thus, the motion of an electron is restricted to those orbits where its angular momentum is an integral multiple of h/2π, where h is Planck’s constant. • Thus we have the relationship mvr = nh/2π, where m is mass of electron, v is velocity of electron of said orbit, r is radius of that orbit, n is a simple integer.
- 11. • The stationary states or allowed energy levels are only those where n = 1, 2, 3, …… This is called Bohr quantum condition. • The energy of an electron changes only when it moves from one orbit to another. An electronic transition from an inner orbit to outer orbit involves absorption of energy. Similarly, when an electron jumps from an outer orbit to inner orbit it releases energy, which is equal to the difference between the two energy levels.
- 13. • The energy thus released in the form of a radiation of a certain frequency appears in the form a line in the atomic spectrum. If the energy of an electron in the outer orbit (n2) is E2 and energy of electron in the inner orbit (n1) is E1 then E2 - E1 = ΔE = hν. • The value of n could be small integers 1, 2, 3 and these correspond to the first, second, third, and so on. Quantum states are shells for the electron; n is termed as principal quantum number.
- 14. • Based on the Bohr theory ,Bohr calculated the radii of the various orbits and the energies associated with the electrons present in those shells.
- 15. Successes of Bohr’s Model • When electron jumps from lower energy level to higher energy level, it absorbs relevant amount of energy and this results in the absorption spectrum.
- 16. • When an electron drops to higher level from lower level, it emits some amount of energy and emission spectrum is observed. • Since there is only one electron in hydrogen atom, there should be one line in hydrogen spectrum. But in Bohr theory, there are infinite number of orbits, so more than one line is observed in spectrum.
- 17. Calculations based on Bohr’s Model • Radius of nth orbit According to bohr model, the attraction force between electron and nucleus is balanced by centrifugal force of electron which is due to motion of electron and tend to take electron away from nucleus.
- 22. • Since the value of Z is constant for an atom, r n α n2 , so radius increases with increasing the value of n. • If the value of n is constant , rn α 1/Z • Hence, radius of a particular orbit decreases with increasing the atomic number.
- 25. • Energy of electron in nth orbit According to Bohr atomic model, the maximum energy value of electron at infinite is zero because of negligible attraction force between electron and nucleus at infinite distance. Hence, as electron comes closer to nucleus, the energy becomes negative.
- 31. Limitations of Bohr’s Model • Bohr model could not explain those atoms which have more than one electron like lithium, helium. This model was applicable only for those atoms which have one electron. • Bohr theory explained only spherical orbits. There was no explanation for elliptical orbits.
- 32. • This model failed to explain Zeeman Effect and stark effect. • Bohr model could not explain the uncertainty principle of Heisenberg.
- 33. • Bohr model was not related with classification and periodicity of elements. • By using Bohr atomic model, one can’t explain the intensity of spectrum line.
- 34. • Bohr model could not explain the wave nature of electron. It explained only particle nature of electron.
- 35. Bibliography • Physical Chemistry, Part-1 by Dr. K.S. Verma • http://en.wikipedia.org/wiki/history of atomic theory
- 36. Thanks for your kind attention