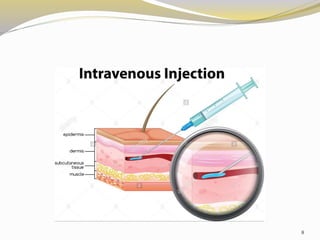
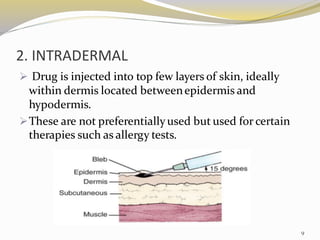
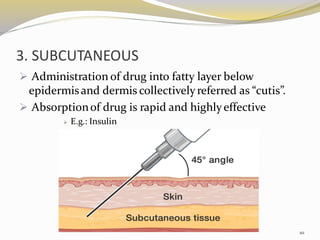
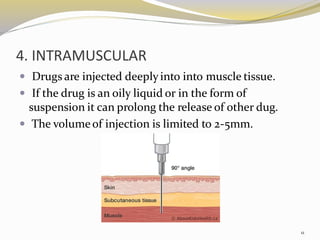
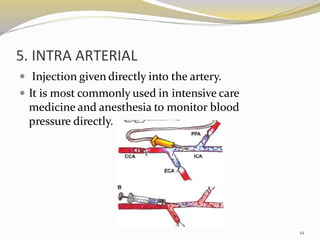
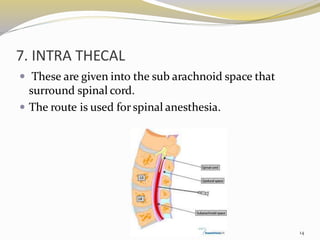
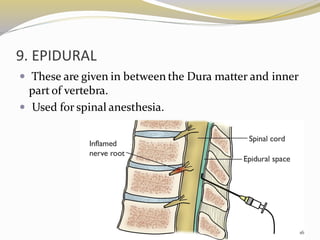
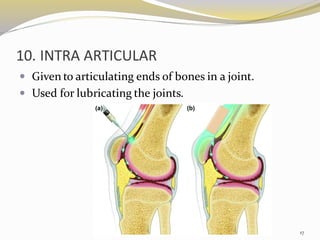
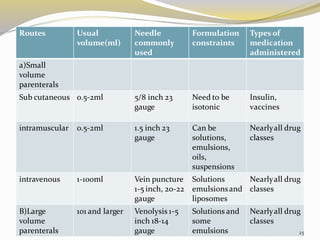
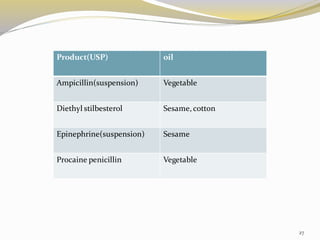
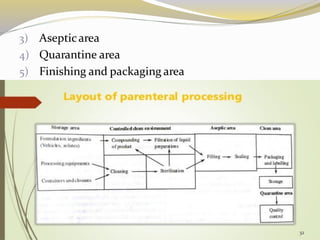
This document provides an overview of parenteral drug formulations and administration. It discusses the definition and routes of parenteral administration including intravenous, intramuscular, subcutaneous, and others. It covers the advantages and disadvantages of the parenteral route. The document categorizes parenteral formulations based on volume into small volume parenterals less than 100mL and large volume parenterals greater than 100mL. It describes the formulation, development, quality control testing, and administration of parenteral drug products.