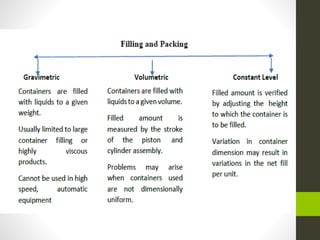
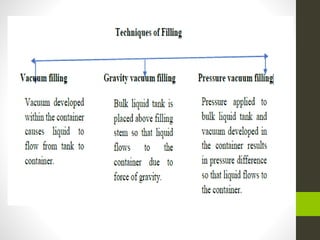
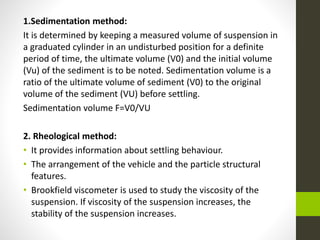
The document provides information on liquid oral dosage forms. It discusses monophasic and biphasic liquid dosage forms, as well as the vehicles, excipients, and formulation considerations involved in producing liquid oral medications. Specifically, it covers emulsions, suspensions, syrups, and elixirs - the main types of liquid oral dosage forms. It also addresses the advantages and disadvantages of liquid dosage forms, as well as best practices for manufacturing, evaluating, and packaging these drug formulations.