Embed presentation
Download to read offline



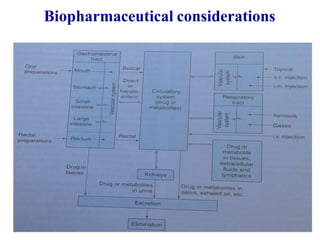


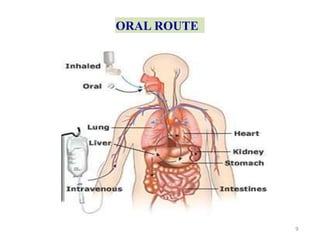
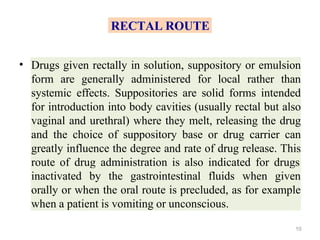

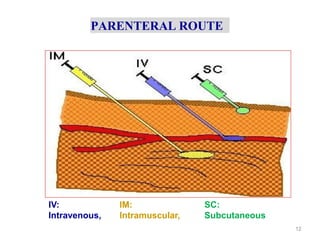


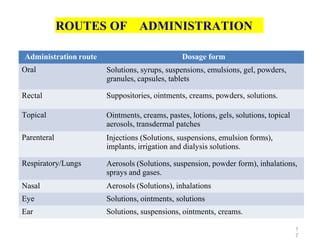





























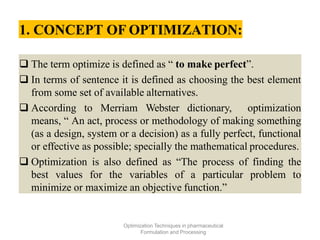


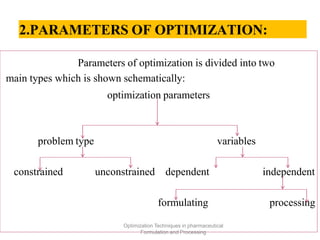
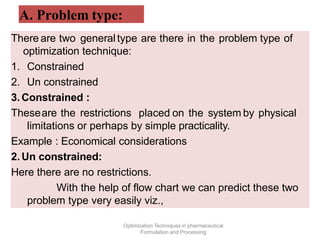




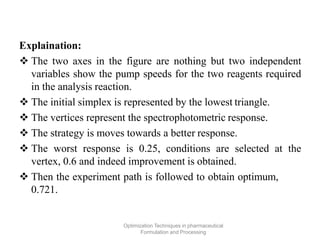
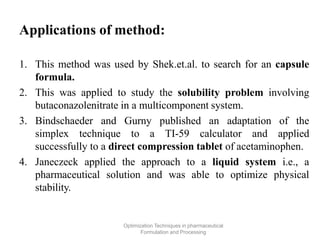

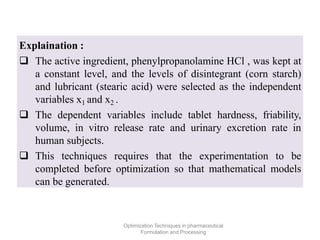
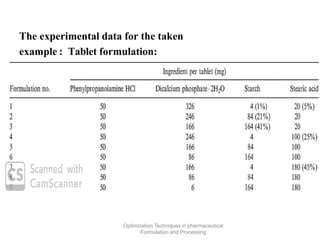






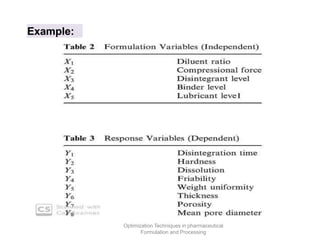

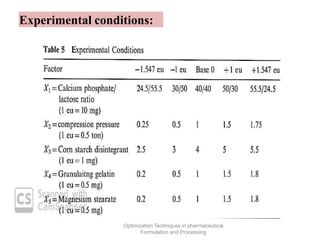
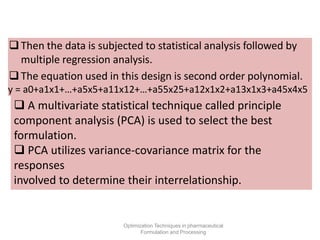
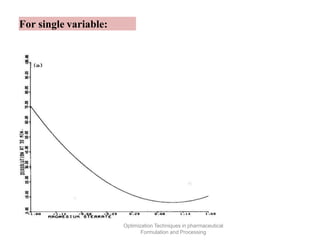
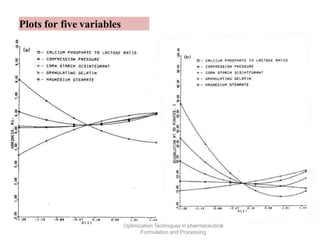

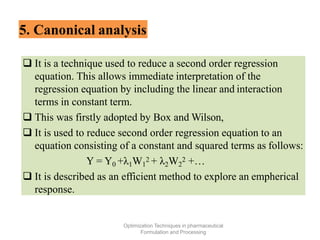

























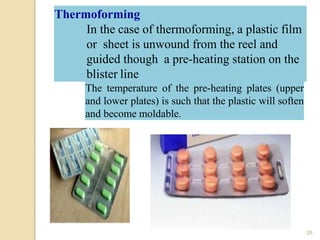





This document discusses key considerations for dosage form design, including: 1. Product development steps like formula and process optimization and packaging selection. 2. Biopharmaceutical factors like drug solubility, dissolution, and routes of administration that influence absorption. 3. Drug properties that impact dosage design such as particle size, solubility, stability, and organoleptic qualities. The goal is to design dosage forms that deliver drug effectively and are acceptable to patients.
























































































































