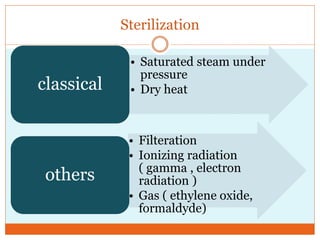
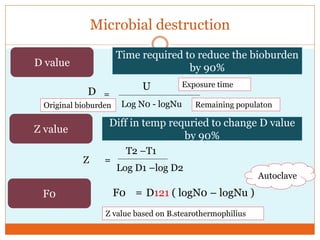
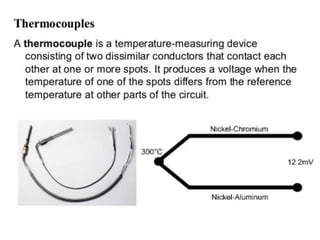
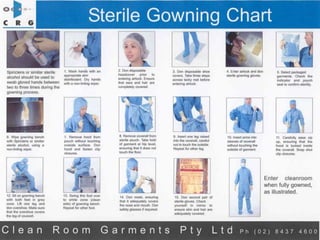
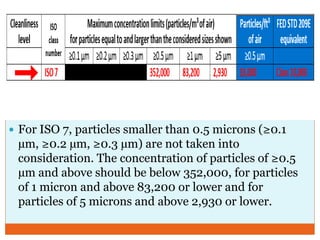
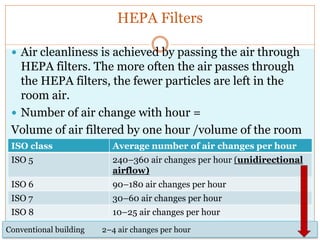
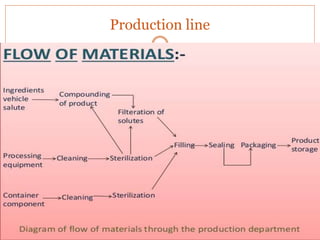
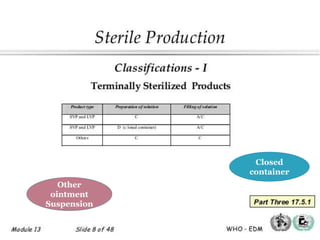
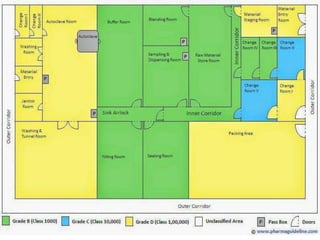
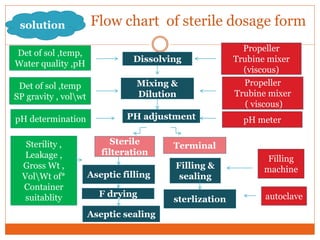
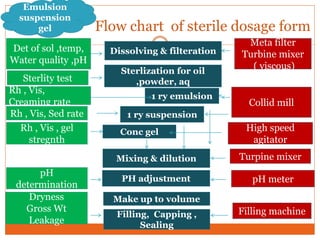
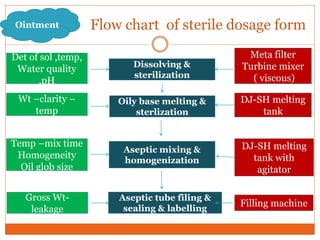
This document discusses the production of sterile dosage forms. It covers topics such as types of sterile dosage forms including parenteral, ophthalmic, and pyrogen-free forms. It also discusses water for injection, types including water for injection, sterile water for injection, and bacteriostatic water for injection. Methods of sterilization are outlined like steam sterilization, dry heat, filtration, and radiation. Other areas covered include air classification systems, HEPA filters, and production line flow charts.