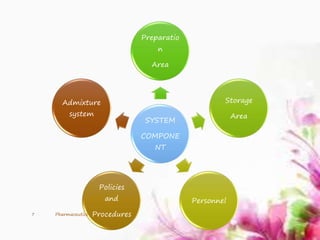
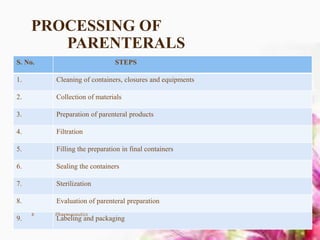
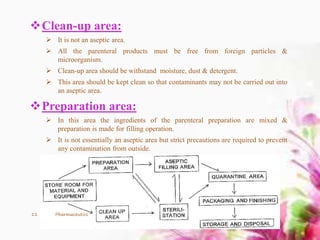
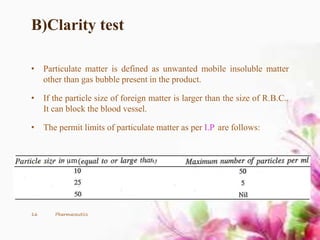
This document provides an overview of the formulation and development of parenteral products. It discusses the key components including containers, closures, processing, formulation, production facilities, and evaluation methods. The production area is divided into five sections - cleanup, preparation, aseptic, quarantine, and finishing/packaging areas. Parenteral formulations contain active drugs, vehicles, and adjuvants. Finished products undergo sterility, clarity, leakage, pyrogen, and assay testing to ensure quality control.