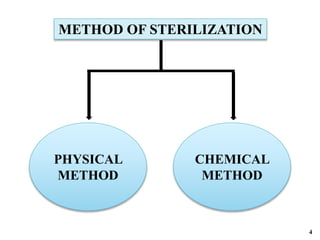
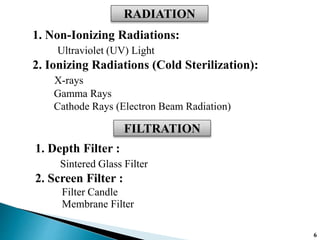
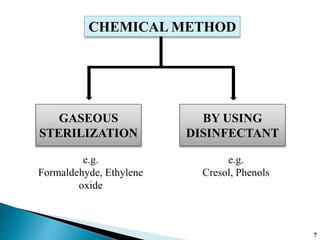
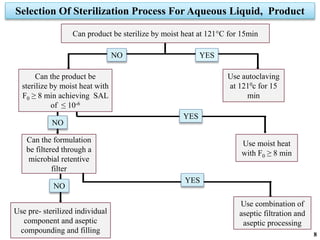
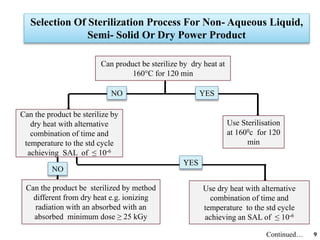
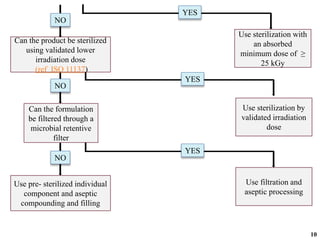
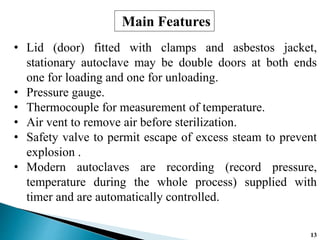
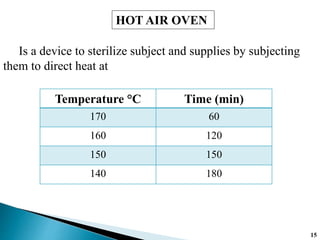
The document covers various methods of sterilization, including physical and chemical techniques, emphasizing the importance of eliminating microorganisms to ensure product safety in pharmaceutical applications. It elaborates on the specifications and validation processes for sterilization equipment, such as autoclaves and dry heat ovens, detailing their operational standards and effectiveness. Additionally, it highlights the regulatory guidelines and ongoing process validation necessary for maintaining product quality throughout manufacturing.