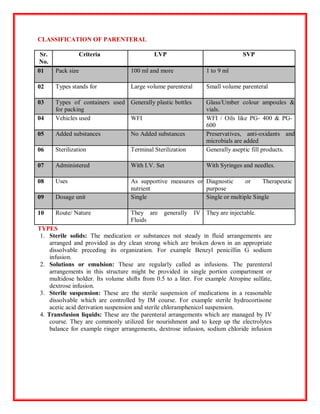
The document outlines essential information about parenteral dosage forms, which are sterile solutions administered through non-oral routes like injections and infusions. It describes the general requirements, advantages, disadvantages, and various types of parenteral administration techniques, along with a detailed process for the preparation and evaluation of these formulations. Additionally, it highlights the importance of maintaining sterility and pyrogen-free conditions during preparation and includes key testing methods for ensuring the quality of parenterals.