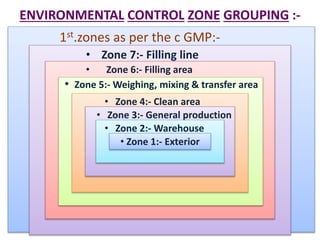
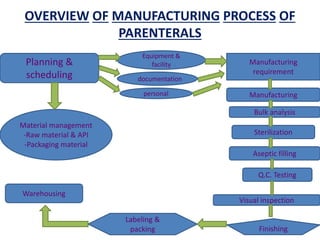
The document discusses the manufacturing process of parenteral preparations. It describes parenterals as sterile liquids or solids for injection or implantation. The manufacturing process involves planning, material management, production, quality control testing, filling, and packaging. Production areas are divided into strict zones based on cleanliness. Environmental controls and facility design aim to prevent contamination, with areas for filling, weighing, storage, and administration. Personnel flow and utility locations are also considered for efficiency.





![[QUALITATIVE LAYOUT OF PARENTERAL MANUFACTURING]
Function
Area
Square meter Percentage
Production 11,094 45.1
Warehouse 7,606 30.9
Utility 1,716 4.1
Quality control 1,716 7.0
Administration 1,018 4.1
Maintenance 1,014 4.5
Employee services 1,014 4.1
Security 39 0.9
Total 24,607 100.0](https://image.slidesharecdn.com/parenteralproduction-140929000858-phpapp01/85/Parenteral-production-7-320.jpg)