The document discusses microbial metabolism and the basic chemical reactions that underlie metabolism. It covers the two major classes of metabolic reactions - catabolism which breaks down molecules and releases energy, and anabolism which uses that energy to build larger molecules. Key metabolic processes like glycolysis, the Krebs cycle, and the electron transport chain are described, which ultimately generate ATP through oxidative phosphorylation or substrate-level phosphorylation. These metabolic pathways allow microbes to acquire nutrients, store energy, build macromolecules, grow and reproduce.

![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.1]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-6-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.2]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-8-320.jpg)

![Basic Chemical Reactions Underlying Metabolism [INSERT TABLE 5.1]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-12-320.jpg)

![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.3]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-15-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT TABLE 5.2]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-16-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.4]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-17-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.5]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-18-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.6]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-19-320.jpg)

![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.7]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-22-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.8]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-23-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.9]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-24-320.jpg)

![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.10]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-26-320.jpg)
![Basic Chemical Reactions Underlying Metabolism [INSERT FIGURE 5.11]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-28-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.12]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-30-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.13]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-34-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.14]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-36-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.15]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-38-320.jpg)
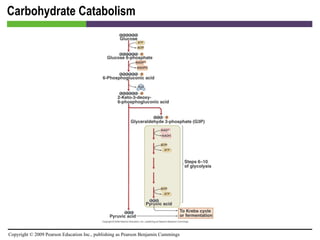
![Carbohydrate Catabolism [INSERT FIGURE 5.17]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-41-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.18]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-45-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.19]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-50-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.20]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-53-320.jpg)
![Carbohydrate Catabolism [INSERT TABLE 5.3]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-57-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.21]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-59-320.jpg)
![Carbohydrate Catabolism [INSERT TABLE 5.4]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-60-320.jpg)
![Carbohydrate Catabolism [INSERT FIGURE 5.22]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-61-320.jpg)
![Other Catabolic Pathways [INSERT FIGURE 5.23]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-64-320.jpg)
![Other Catabolic Pathways [INSERT FIGURE 5.24]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-65-320.jpg)

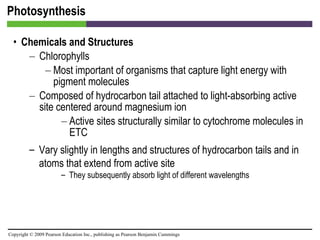
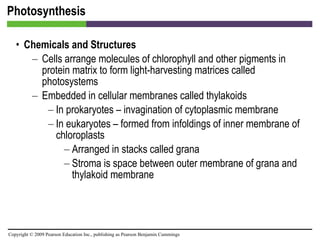
![Photosynthesis [INSERT FIGURE 5.25]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-70-320.jpg)
![Photosynthesis [INSERT FIGURE 5.26]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-73-320.jpg)
![Photosynthesis [INSERT FIGURE 5.27]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-74-320.jpg)
![Photosynthesis [INSERT TABLE 5.5]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-77-320.jpg)
![Photosynthesis [INSERT FIGURE 5.28]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-79-320.jpg)
![Other Anabolic Pathways [INSERT TABLE 5.6]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-82-320.jpg)
![Other Anabolic Pathways [INSERT FIGURE 5.29]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-83-320.jpg)
![Other Anabolic Pathways [INSERT FIGURE 5.30]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-84-320.jpg)
![Other Anabolic Pathways [INSERT FIGURE 5.31]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-85-320.jpg)
![Other Anabolic Pathways [INSERT FIGURE 5.32]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-86-320.jpg)
![Integration and Regulation of Metabolic Function [INSERT FIGURE 5.33]](https://image.slidesharecdn.com/startherech05lecture-100519102635-phpapp02/85/Start-here_ch05_lecture-90-320.jpg)
