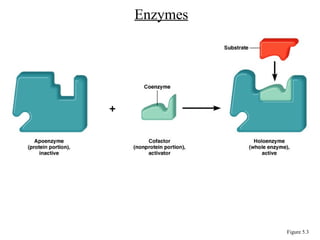
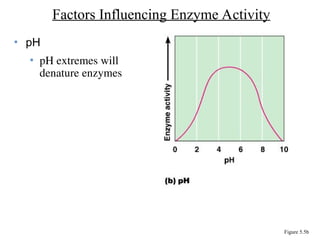
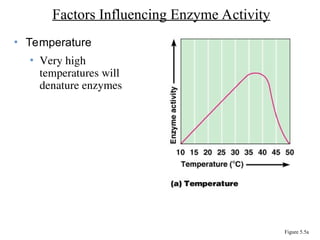
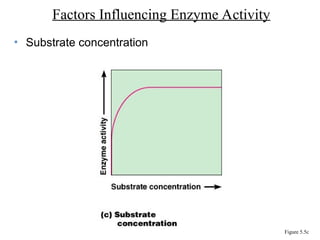
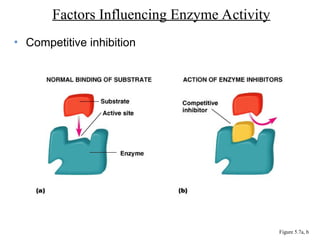
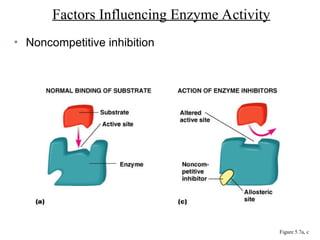
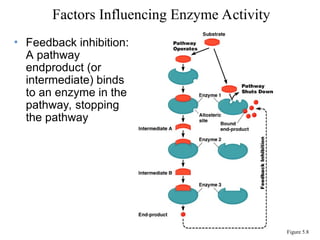
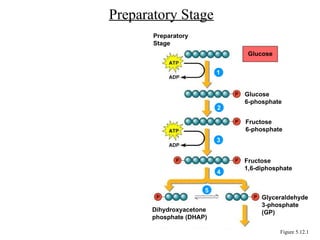
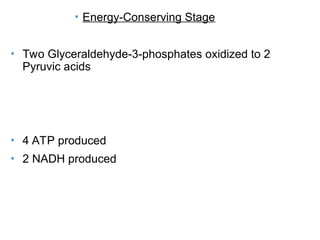
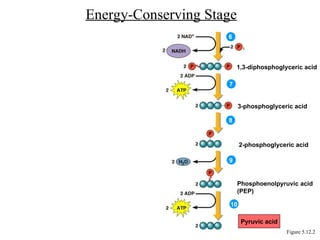
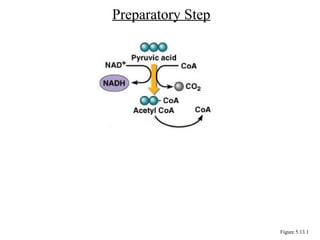
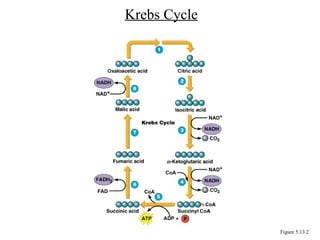
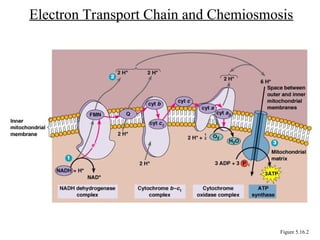
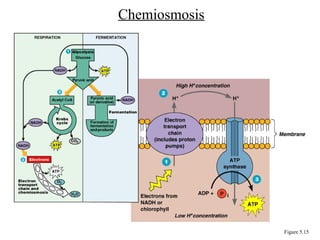
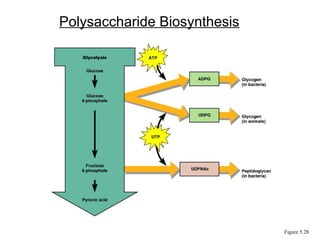
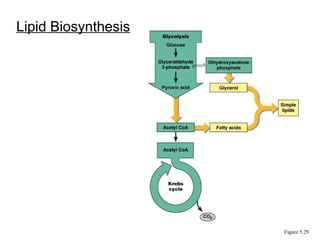
Chapter 5 discusses microbial metabolism, including the processes of energy release and use, ATP generation, and enzyme activity. It details carbohydrate catabolism through glycolysis, the Krebs cycle, and electron transport chain, leading to cellular respiration and fermentation pathways. The chapter also covers metabolic diversity among autotrophs and heterotrophs, along with biosynthesis of polysaccharides and lipids.