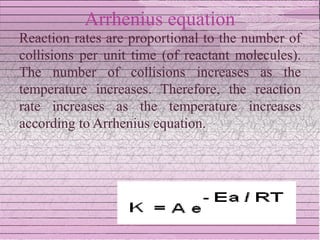
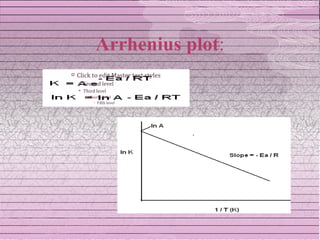
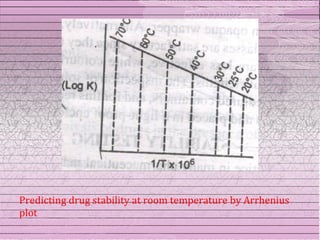
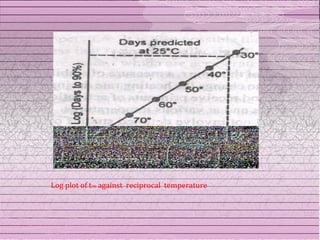
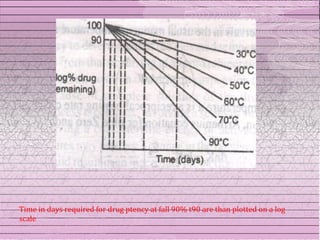
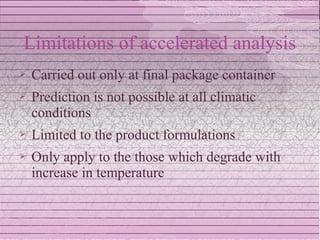
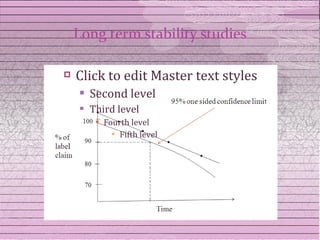
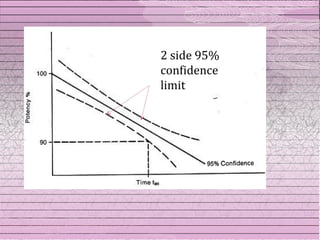
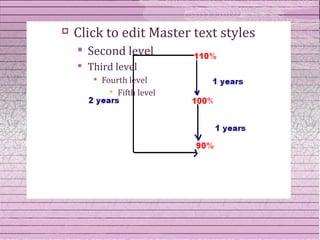
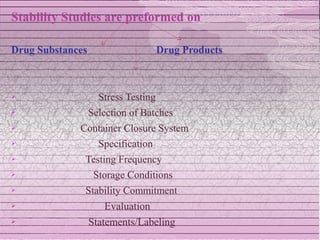
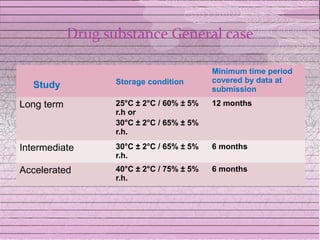
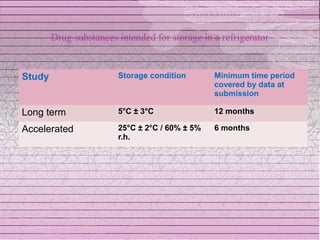
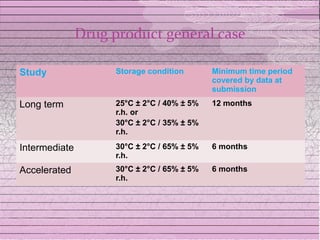
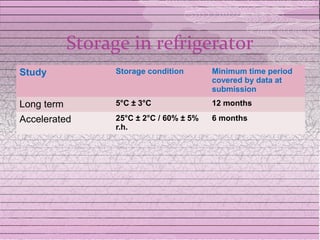
This document discusses drug stability and stability testing. It defines stability as the extent to which a product retains its properties over time. Stability testing is necessary to determine shelf life, recommended storage conditions, and ensure safety. There are various types of stability including chemical, physical, and microbiological. Testing is conducted for different formulations like tablets, capsules, emulsions, and involves evaluating attributes like appearance, assay, degradation products, and more. Guidelines provided by ICH help harmonize stability testing globally.