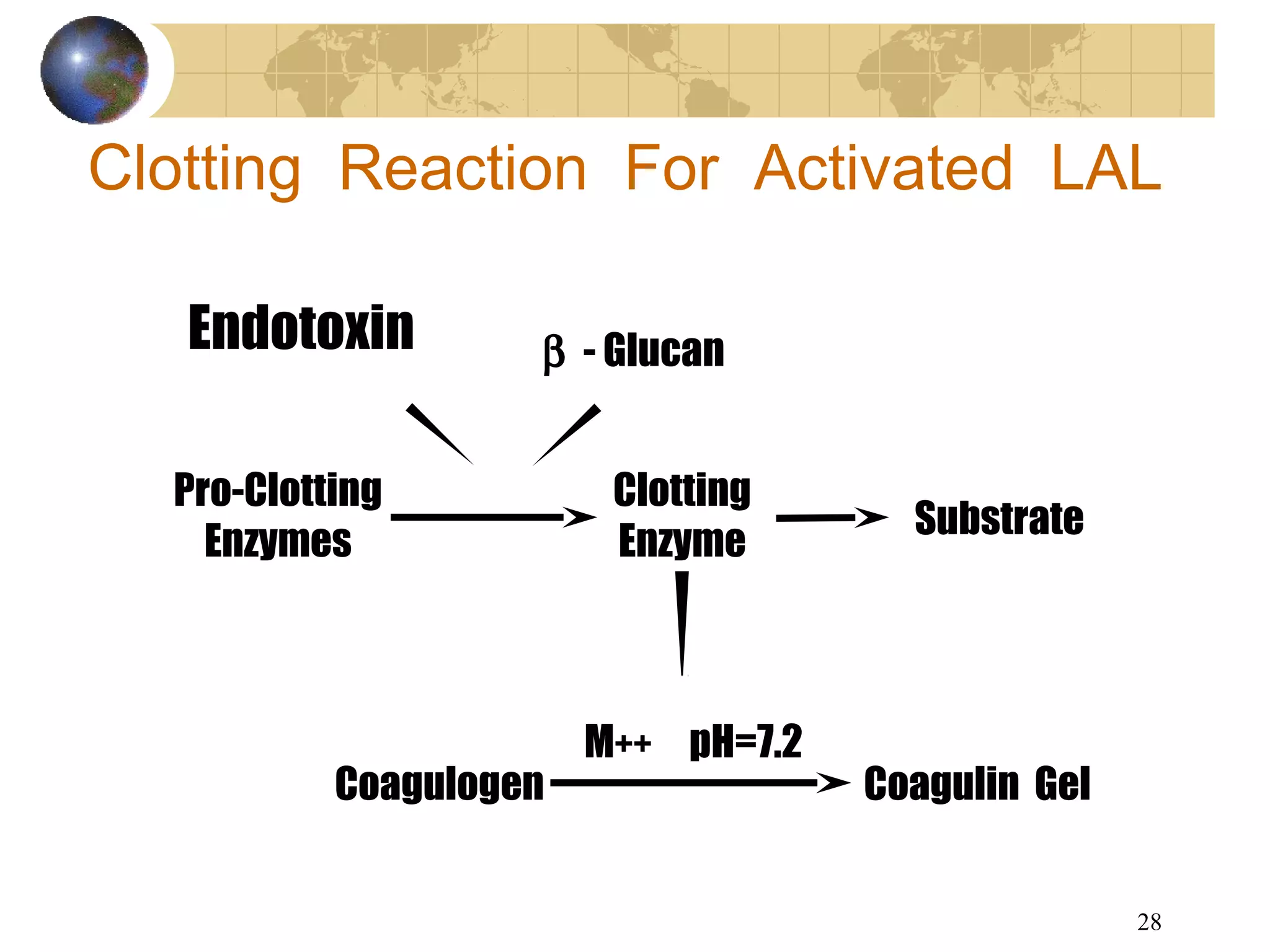
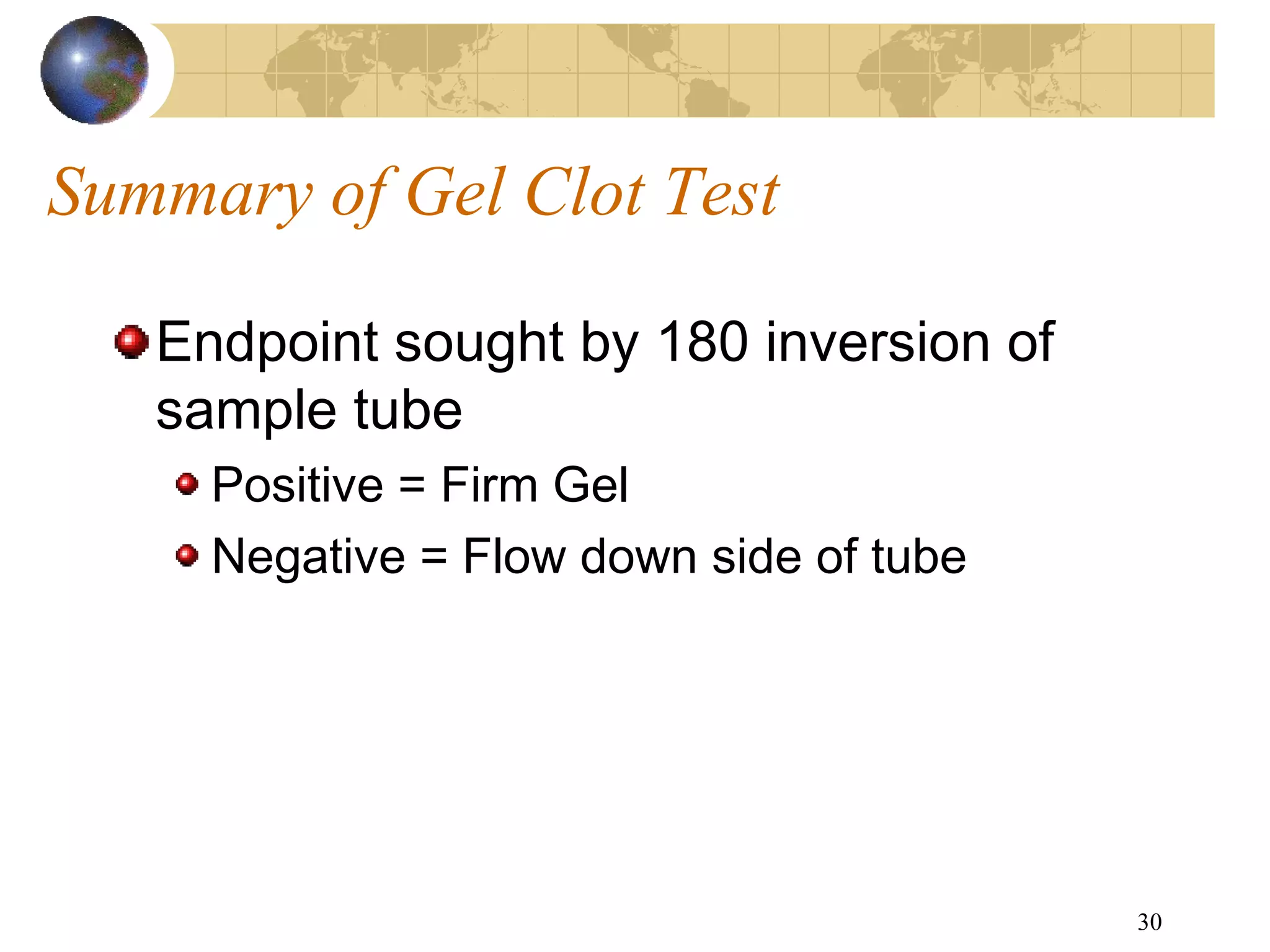
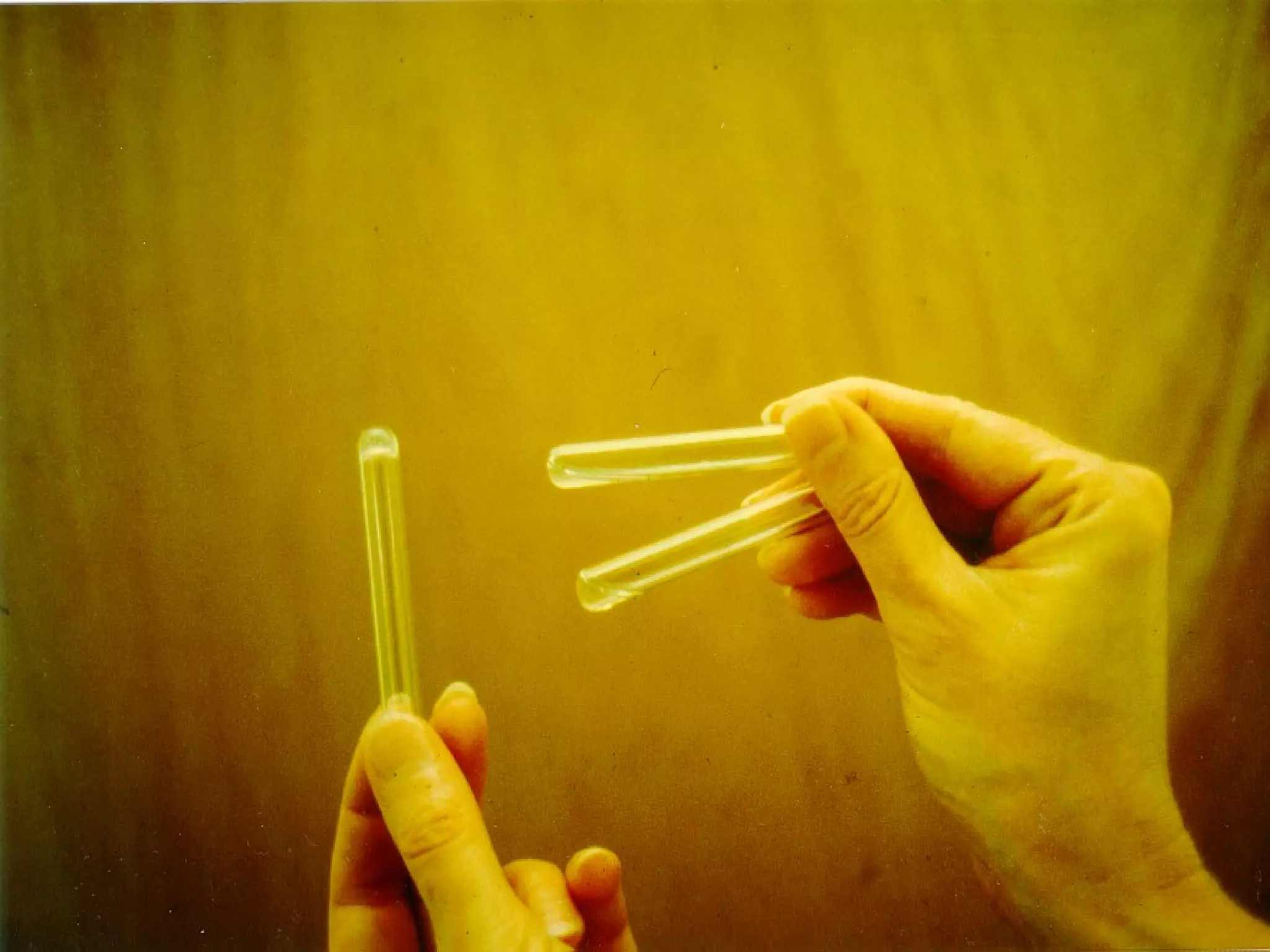
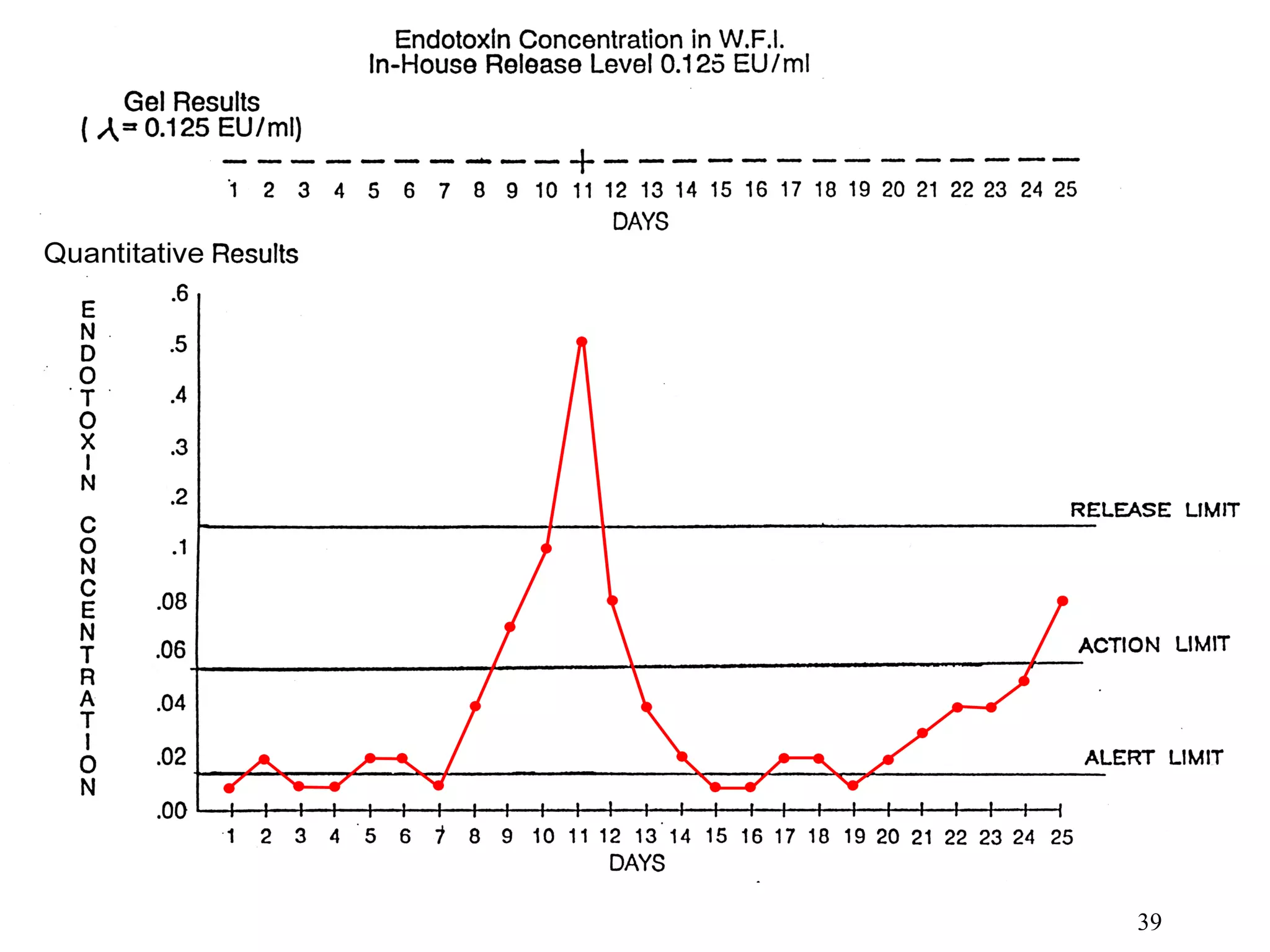
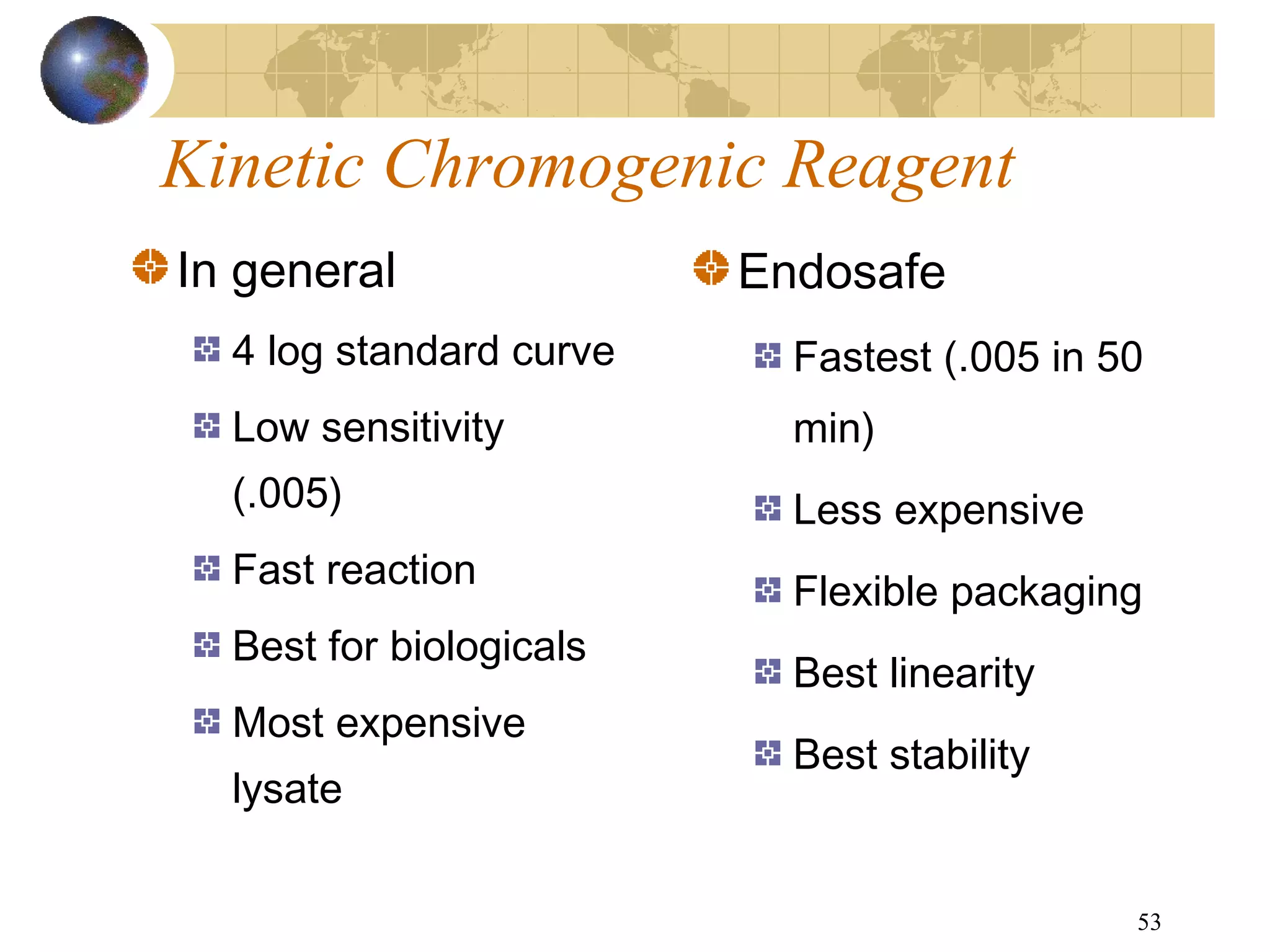
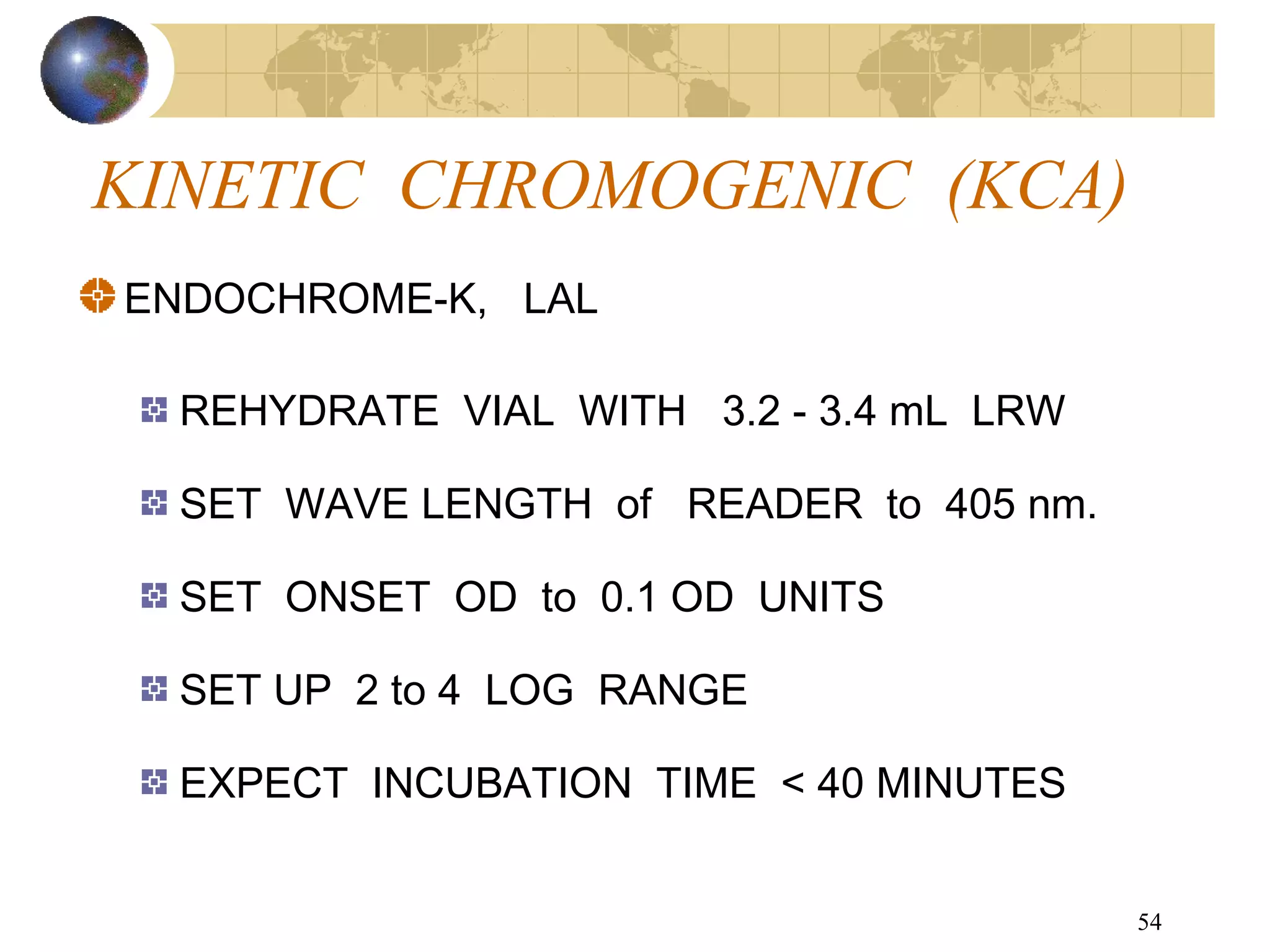
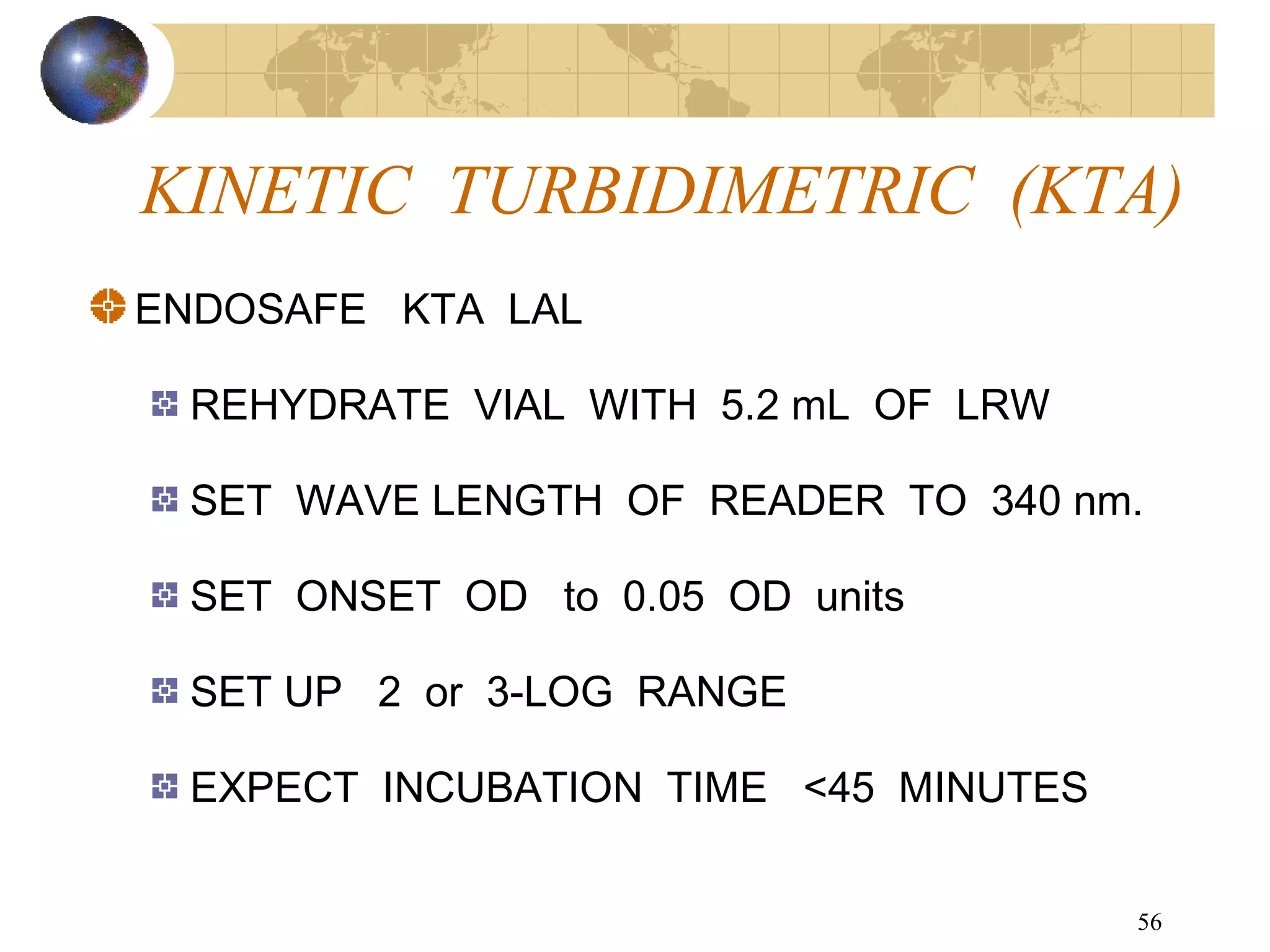
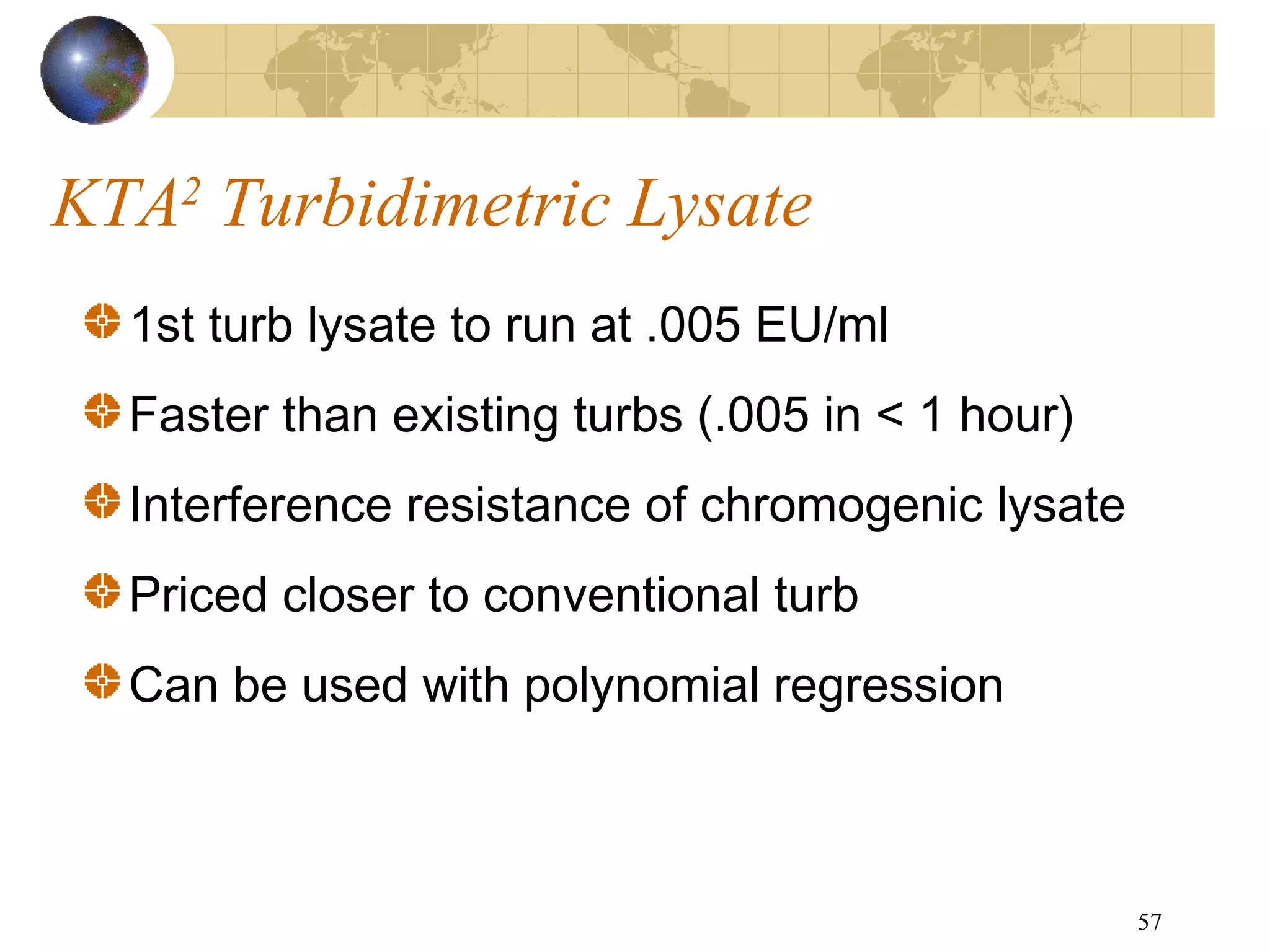
The document discusses the importance of endotoxin testing in parenteral products to prevent adverse reactions, detailing methods like the Limulus Amebocyte Lysate (LAL) assay. History and regulatory evolution of LAL testing are highlighted, including various testing techniques and their advantages, disadvantages, and applications in pharmaceuticals, medical devices, water systems, and dialysis. It emphasizes the necessity for standardized testing procedures and compliance with FDA and USP guidelines to ensure product safety and efficacy.