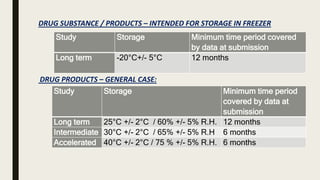
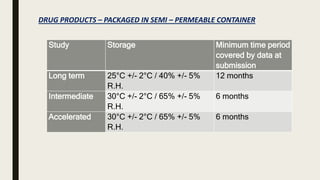
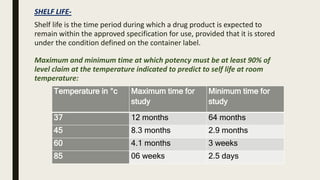
The document discusses ICH stability testing guidelines for drug substances and products, outlining the types of studies required including long term, intermediate, and accelerated studies under various storage conditions. Key aspects that are evaluated include physical, chemical, and microbial changes that may occur over time and factors that influence stability such as temperature, humidity, and light exposure. The purpose of stability testing is to establish a product's shelf life and ensure it remains safe and effective when stored as recommended.