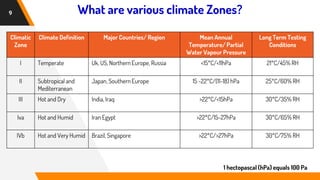
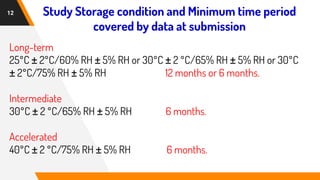
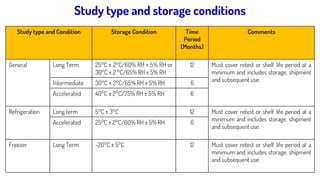
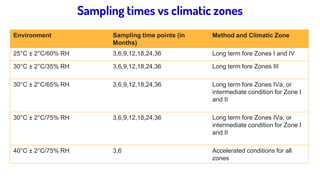
The document discusses stability testing guidelines for pharmaceutical dosage forms, emphasizing the importance of stability in maintaining drug properties during storage and usage. It outlines various types of stability testing including long-term, intermediate, and accelerated stability, as well as the principles and protocols involved in conducting these tests. Additionally, it details climatic zones and their impact on stability testing conditions, along with the significance of photostability evaluation.