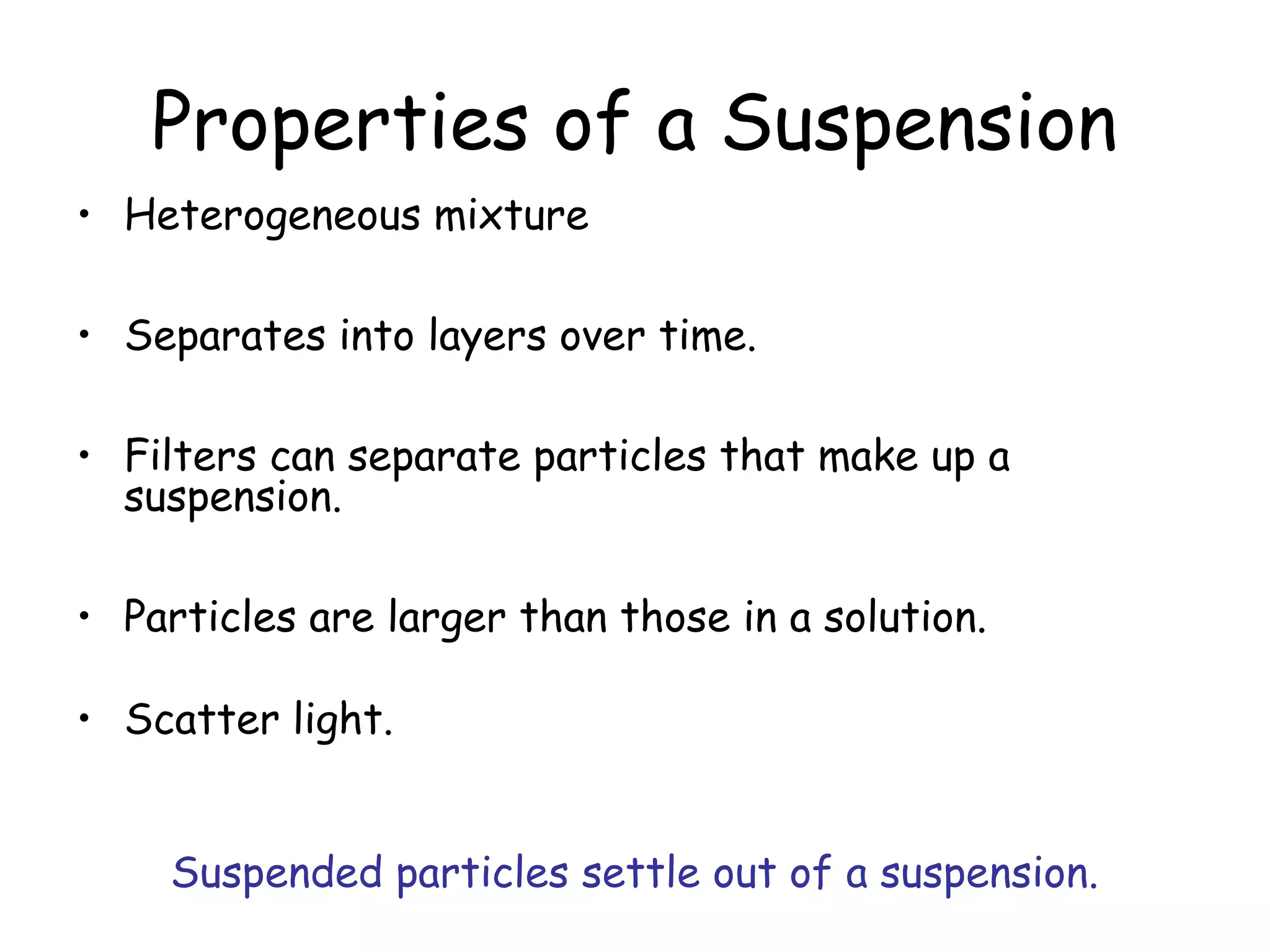
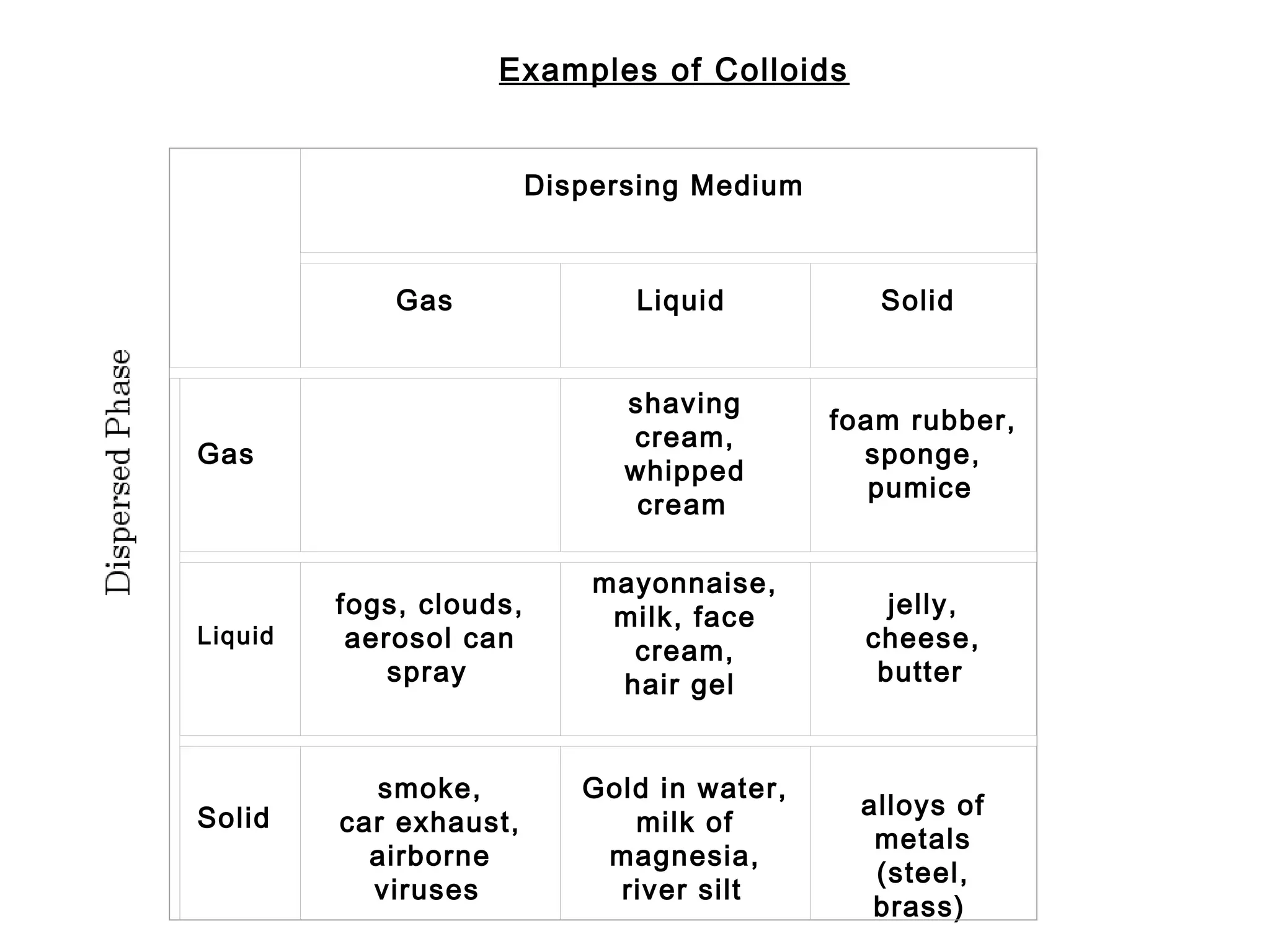
This document discusses the classification of mixtures as solutions, suspensions, or colloids based on particle size. Solutions are homogeneous mixtures where particles are too small to scatter light or be filtered out. Suspensions are heterogeneous mixtures where particles are large enough to separate over time by settling or being filtered. Colloids have intermediate particle sizes that do not separate but can scatter light.