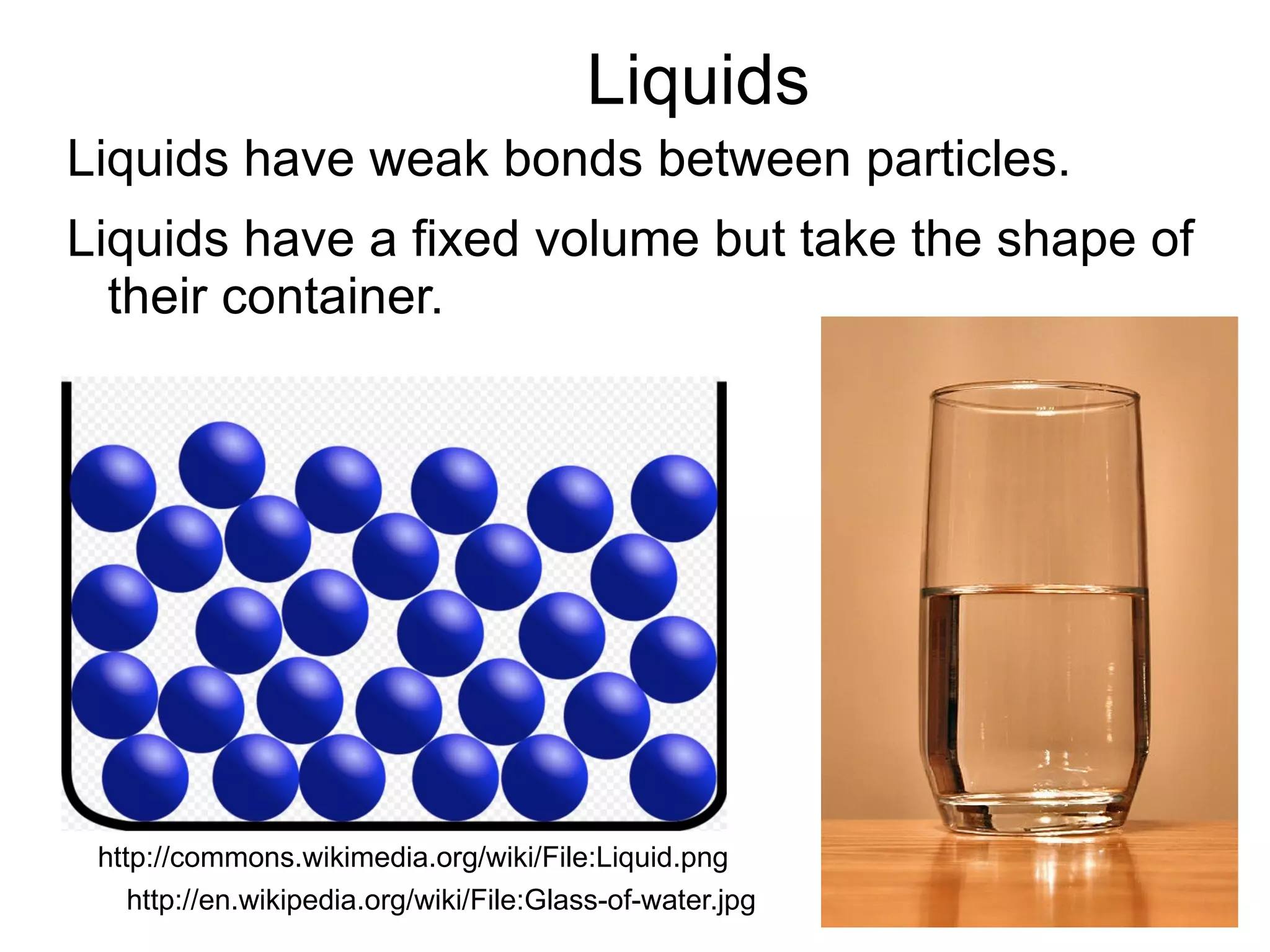
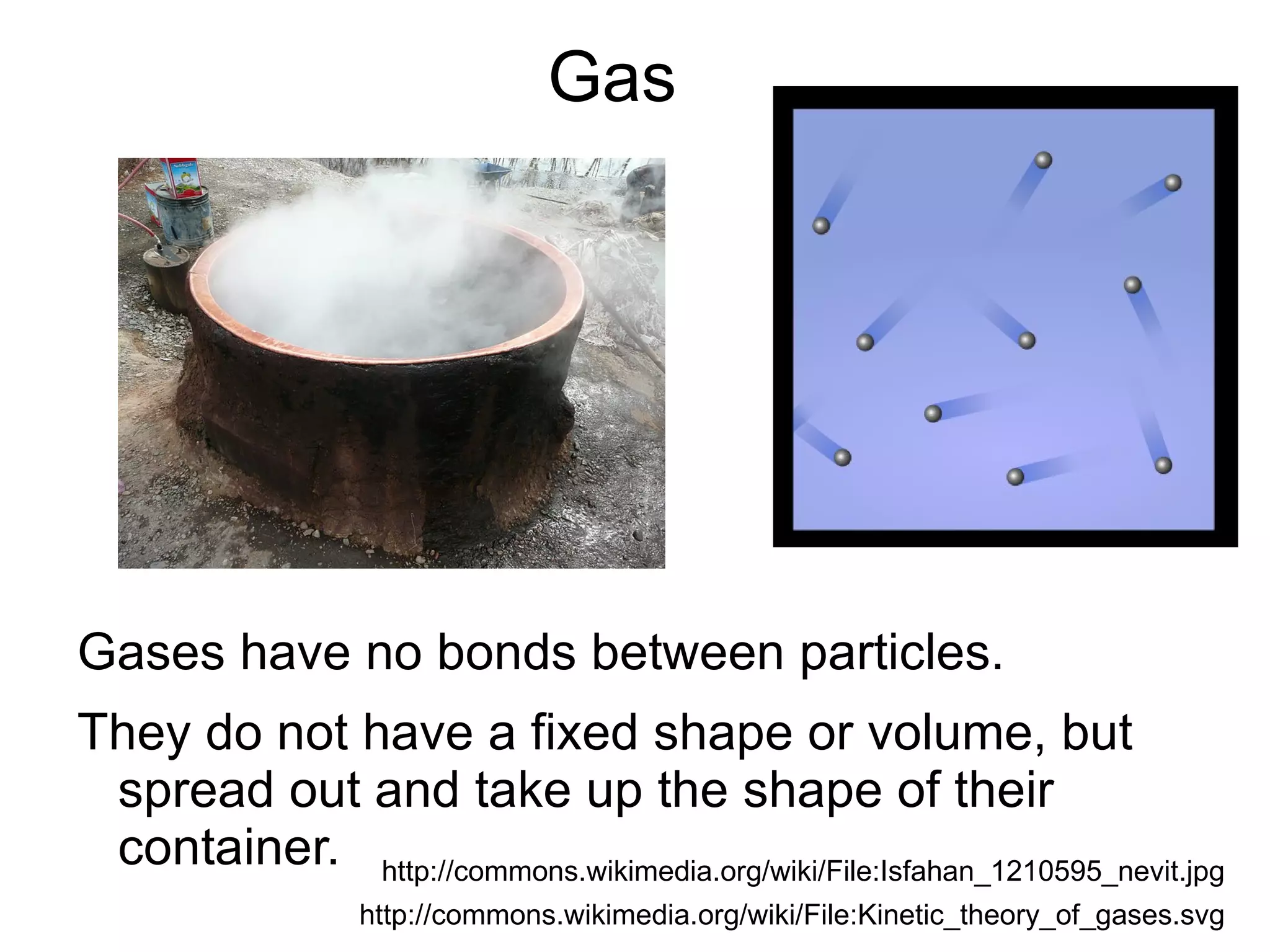
The document discusses the three states of matter: solids, liquids, and gases. It provides descriptions of their characteristic properties - solids have a fixed shape and volume, liquids have a fixed volume but take the shape of their container, and gases spread out and take the shape of their container. Examples are given to illustrate each state of matter. The document also covers topics like diffusion, the relationship between diffusion and temperature, and particle theory to explain states of matter.