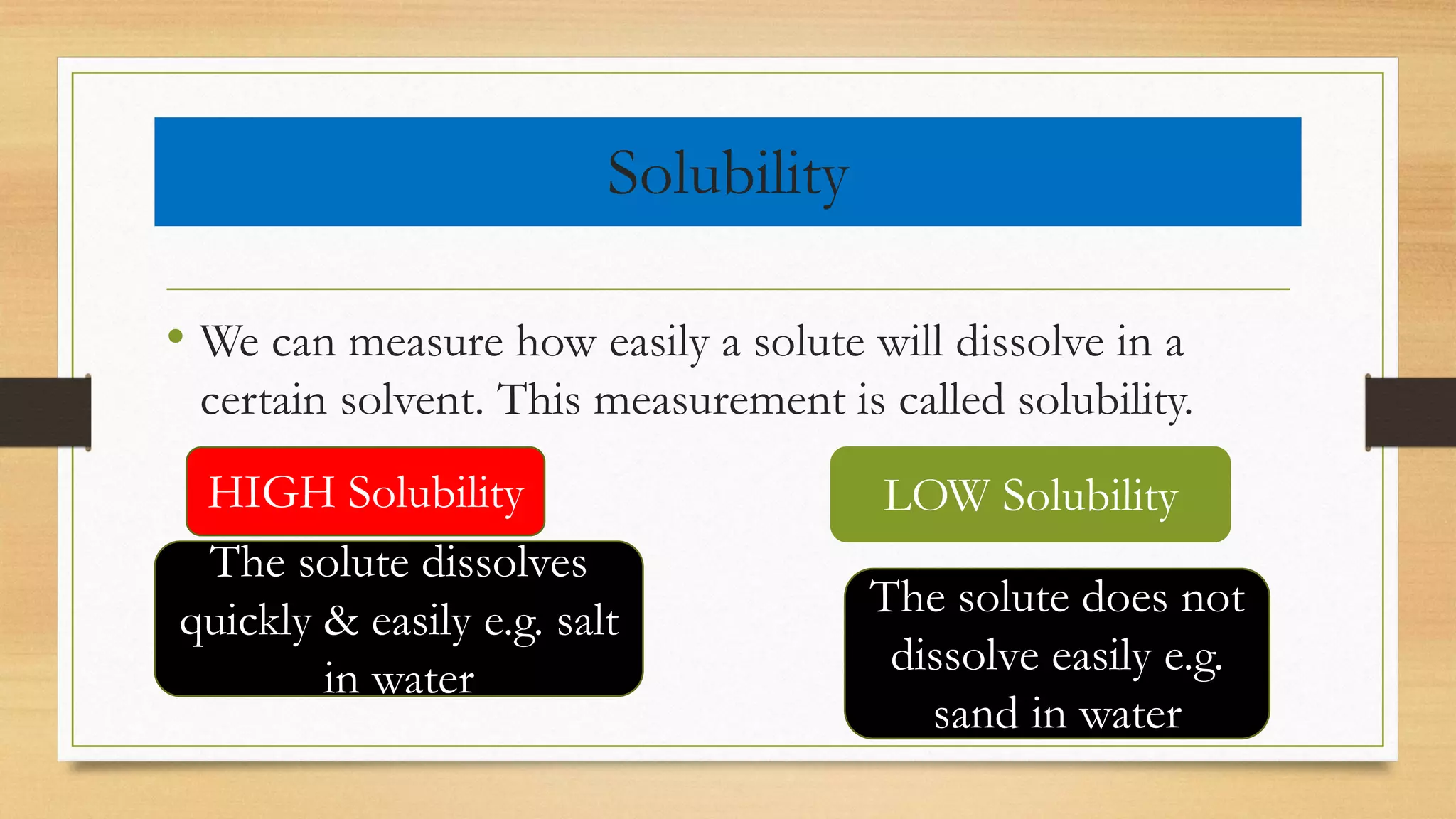
The document provides an overview of mixtures and solutions, defining mixtures as combinations of two or more atoms that are not chemically bonded. It categorizes mixtures into homogeneous and heterogeneous types, with solutions being a special category of homogeneous mixtures comprising a solute and a solvent. Additionally, it discusses solubility, concentration levels (dilute vs. concentrated), and the concepts of saturated and super-saturated solutions.