This document discusses mixtures and separation processes. It covers:
1. Methods to separate solid mixtures including magnetic attraction, filtration, and evaporation.
2. Separation of immiscible liquid mixtures using a separating funnel.
3. Definitions of solution, solute, and solvent. A solution is a mixture where a solute dissolves and spreads through a solvent.
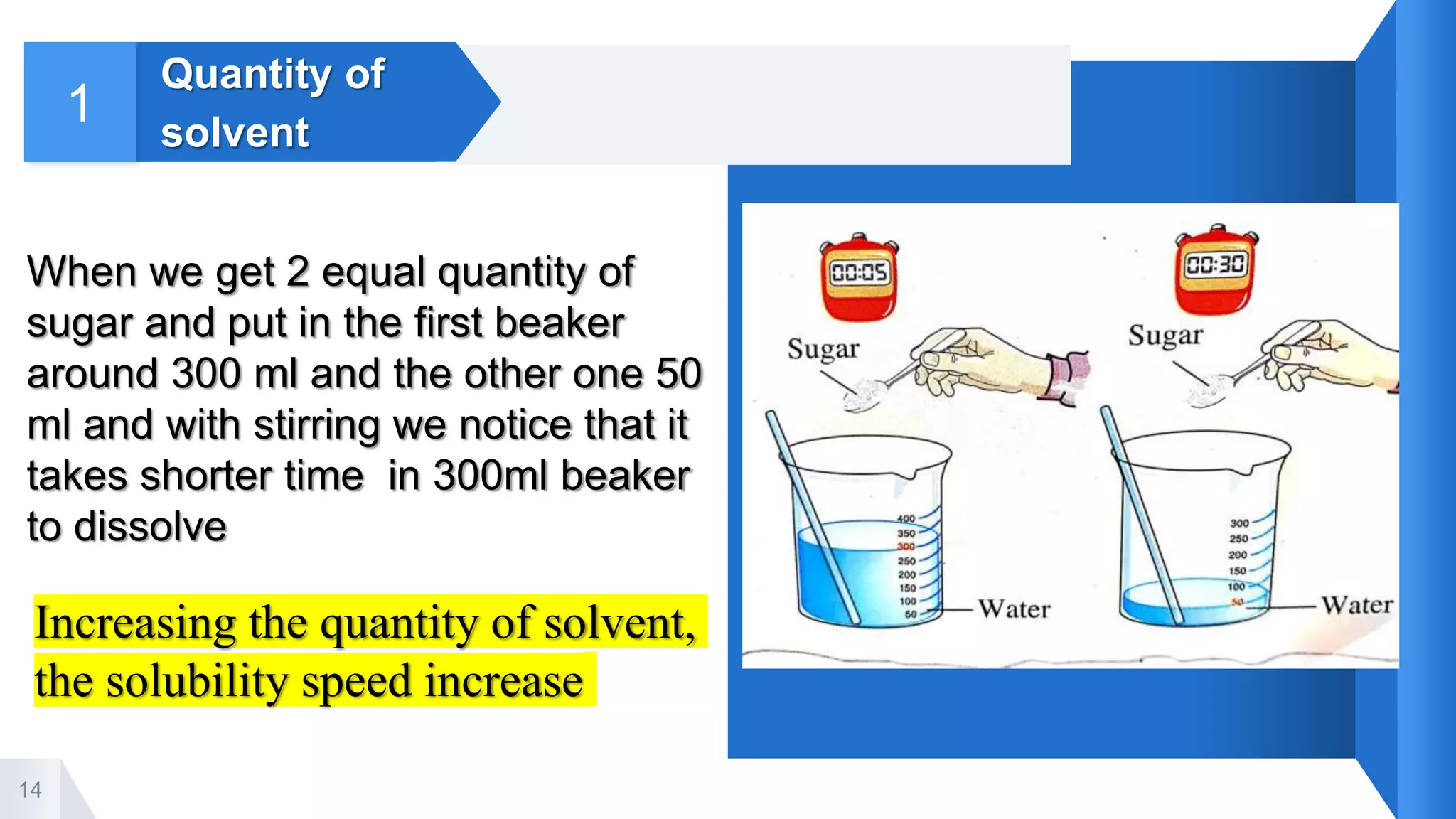
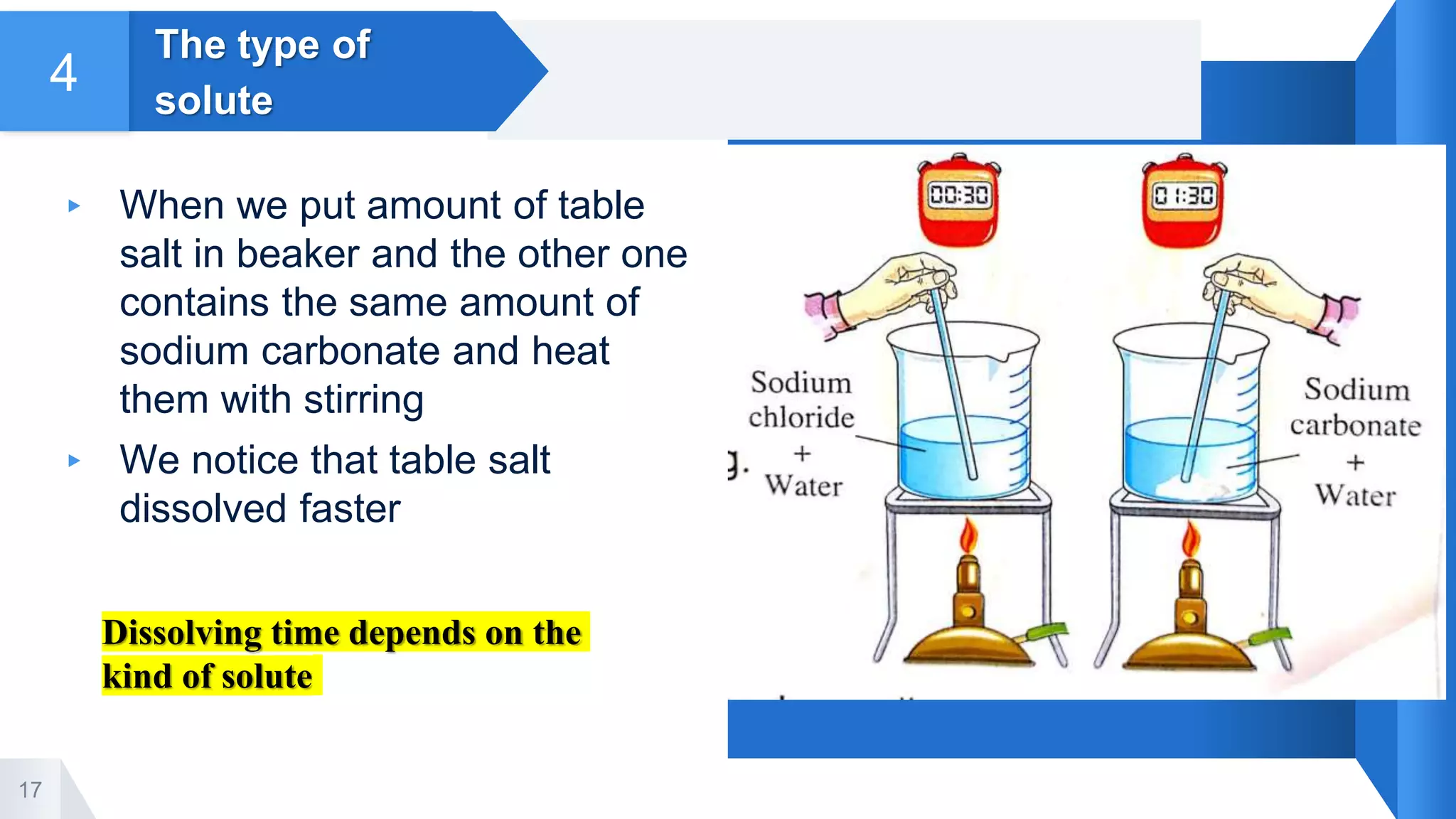
4. Factors that affect the solubility process including quantity of solvent, temperature, stirring, and type of solute. Increasing these factors can increase the speed of dissolution.