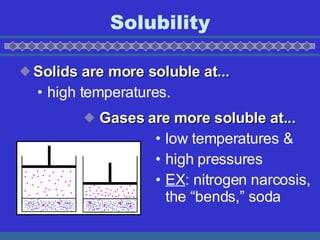
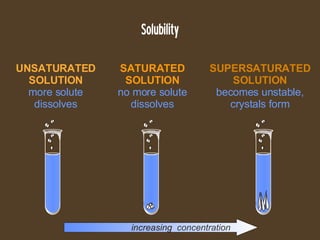
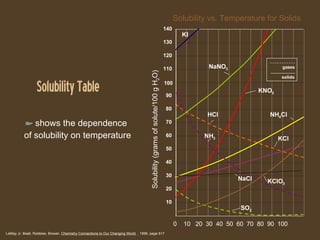
The document discusses solubility and dissolution rates. It defines solubility as the ability of a substance to dissolve in another, and discusses how "like dissolves like" with polar substances dissolving in polar solvents and nonpolar in nonpolar. It also discusses how to increase dissolution rates, such as by increasing temperature, surface area, or movement. For gases, dissolution is increased at lower temperatures and higher pressures. Concentration of solutions is also covered, defining unsaturated, saturated, and supersaturated solutions based on how close they are to the maximum solubility for a given temperature.