The document discusses different types of mixtures. It defines a mixture as being made up of two or more substances that are not chemically combined with each other. Mixtures can be prepared in many different ways and there are both naturally occurring mixtures as well as human-made mixtures. Some key points made include:
- Mixtures have varying compositions and properties depending on how they are prepared
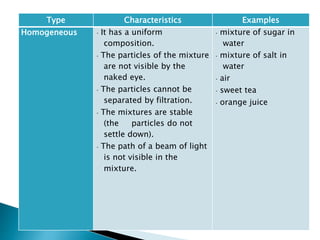
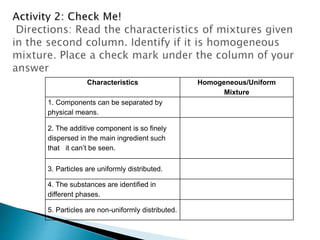
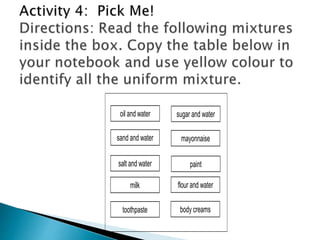
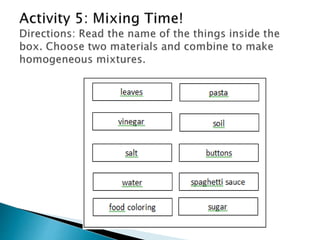
- Both homogeneous/uniform mixtures and heterogeneous/non-uniform mixtures are discussed
- Examples of different types of mixtures are provided like solutions, suspensions, emulsions, and colloids.