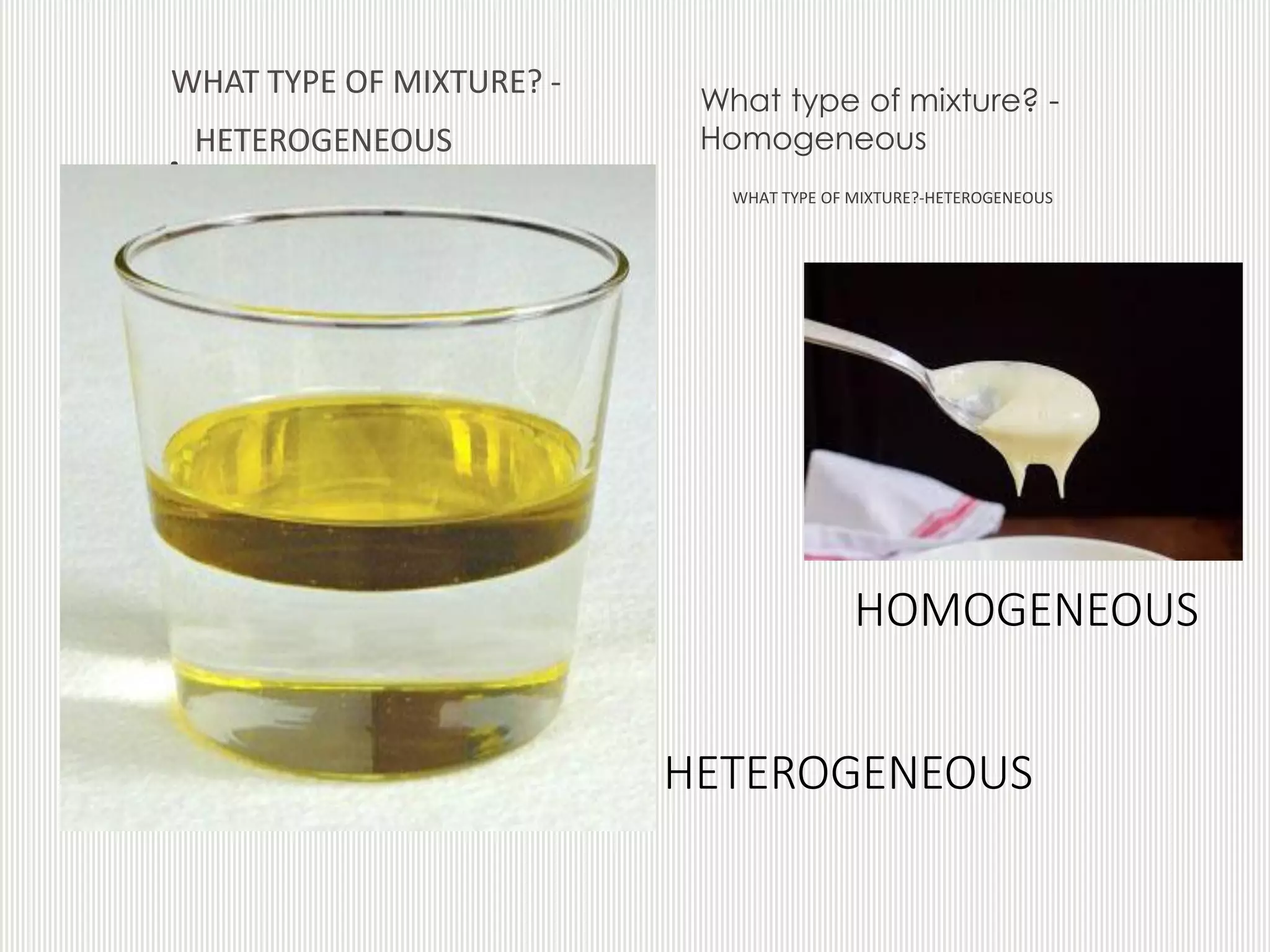
The document discusses different types of mixtures including homogeneous and heterogeneous mixtures, solution-based mixtures, and solid mixtures. Homogeneous mixtures have a uniform composition while heterogeneous mixtures have a non-uniform composition. Solution-based mixtures can be classified as true solutions, colloidal solutions, or suspensions depending on the particle size of the solute. Solid mixtures include alloys, which are homogeneous solid solutions of metals that are difficult to separate. An example given is brass, which is a homogeneous mixture of copper and zinc that takes on properties between the two metals.





![Sieving; Free Image Courtesy:
http://www.ingridscience.ca/n
ode/338
Hand picking; Free Image
Courtesy:
www.shutterstock.com
Gravity Separation. Free Image Courtesy:
https://www.911metallurgist.com/blog/grav
ity-spiral-separator-working-principle/how-
spirals-work#iLightbox[postimages]/0
Magnetic Separation; Free Image Courtesy:
https://infograph.venngage.com/p/44403/separation-
methods; Right: http://www.glogster.com/favianheng/separation-
of-mixtures-prss-1e5-chemistry-/g-6ms3bthoq6926283cl6iua0
Sorting Machine - Skittles
and M&M's
https://www.youtube.com
/watch?v=tSEHDBSynVo
Winnowing: Free Image Courtesy:
Alamy Stock Photos
Froth Flotation: Free
Image
Courtesy:
https://www.911metall
urgist.com/blog/mineral
-processing-froth-
flotation](https://image.slidesharecdn.com/hetrogenousandhomogenousmixtureppt-170820162448/75/Hetrogenous-and-homogenous-mixture-ppt-6-2048.jpg)