The document discusses the color of transition metal complexes. It explains that transition metal complexes are colored due to d-d electron transitions when visible light is absorbed. The color depends on the metal ion, ligands, and crystal field splitting energy between d-orbital sets. Complexes with partially filled d-orbitals absorb specific wavelengths of light and appear colored, while those with empty or filled d-orbitals do not undergo d-d transitions and appear colorless. Examples are given of different complexes and the colors they appear based on the wavelengths they absorb.



![Characteristics of absorbed light
The absorbed light has the following characteristics:
i) Colours of the absorbed and transmitted light
• Since white light is composed of many different colours, the
colours of the absorbed light and transmitted light are different
from each other.
• The colour of the transmitted light is called complementary
colour of the absorbed light (see following table).
• As a matter of fact, the colour of a given complex ion or
compound is the colour of the transmitted light.
• For example:
a) Since hydrated Cu2+ ion, i.e., Cu2+ ion present in
[Cu(H2O)5]SO4 or CuSO4.5H2O absorbs yellow radiation, it
transmits blue radiation and hence this ion looks blue to our
eyes.
[Cu(H2O)5]SO4 or CuSO4.5H2O
Cu2+ ion → Yellow radiation → Blue](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-5-320.jpg)
![b) Hydrated Ti3+ ion, i.e., Ti3+ ion present in [Ti(H2O)6]3+ absorbs
green radiation and hence transmits the radiation of purple
colour.
• Hydrated Ti3+ ion, therefore, looks violet (almost purple).
c) Anhydrous Co2+ compounds absorb the radiation of red colour
and therefore appear blue green.
• On the other hand the hydrated Co2+ ion, i.e., Co2+ ion in
[Co(H2O)6]2+ ion, absorbs blue green radiation and therefore,
appears red.
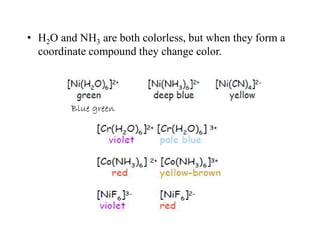
• The colours of some common hexahydrated transition metal
complex ions, [M(H2O)6]n+ given in following table.
[Ti(H2O)6]3+
Ti3+ ion → Green → Purple
Anhydrous Co2+
Co2+ ion → Red → Blue green
Hydrated Co2+
Co2+ ion → Blue green→ Red](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-9-320.jpg)
![Table: Colours of some hexahydrated transition metal complex
ions, [M(H2O)6]n+. For the sake of convenience, 6H2O molecules
have not been shown
(n = No. of unpaired electrons).](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-10-320.jpg)
![ii) Nature of the central metal atom/ion
• The colour of the absorbed light and hence that of the complex ion
or compound depends on the nature of the central metal atom/ion.
• For example Ti3+ ion in [Ti(H2O)6]3+ absorbs green radiation and
hence [Ti(H2O)6]3+ ion looks violet (almost purple).
• On the other hand Co2+ ion in [Co(H2O)6]2+ ion absorbs blue green
radiation and, therefore, appears red.
• Thus we see that although Ti3+ arid Co2+ ions are attached with the same
ligands (viz. 6H2O molecules), they absorb the radiations of different
colour and hence [Ti(H2O)6]3+ and [Co(H2O)6]2+ ions appear different in
their colour.
iii) Nature of the ligands
• The colour of the absorbed light also depends on the nature of the ligands
• For example Ni2+ ion in [Ni(NH3)6]2+ ion absorbs yellow radiation and
hence has blue colour.
[Ti(H2O)6]3+
Ti3+ ion → Green → Purple
[Co(H2O)6]2+
Co2+ ion → Blue green→ Red](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-11-320.jpg)
![• On the other hand Ni2+ ion in [Ni(H2O)6]2+ ion absorbs red colour and
is, therefore, blue green in colour.
• Thus, we see that although the central metal ion is the same in both the
complex ions, these ions absorb radiations of different colours and,
therefore, have different colours.
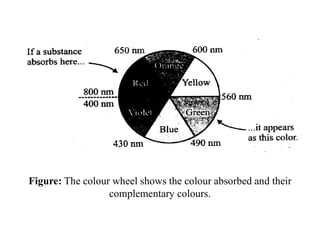
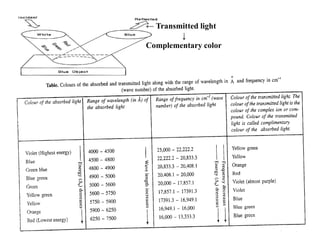
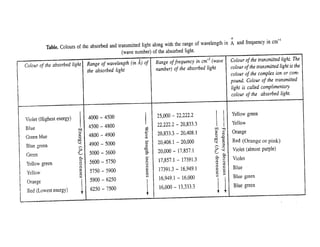
iv) Wavelength of the absorbed light
• The wavelength of the absorbed light lies in the visible region of the
electromagnetic radiation (4000 - 8000 Å), i.e., the transition metal
ions absorb only that portion of the incident light whose wavelength
lies in between 4000Å and 8000Å (visible region).
v) Energy calculation
• The energy associated with the wavelength of the radiation absorbed
by the complex ion can also be calculated.
• Suppose [M(H2O)6]n+ ion which is a complex ion absorbs the radiation
whose wavelength is equal to λÅ.
[Ni(NH3)6]2+
Ni2+ ion → Yellow → Blue
[Ni(H2O)6]2+
Ni2+ ion → Red → Blue green](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-12-320.jpg)
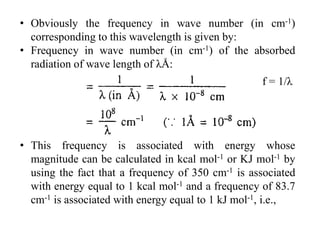
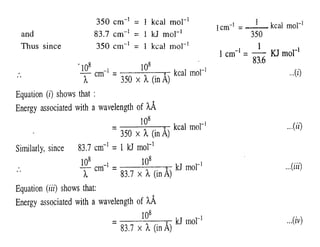
![• Equation (ii) and (iv) both show that the energy associated
with the absorbed radiation is inversely proportional to
the wavelength of the radiation but directly proportional
to the frequency of the radiation.
To explain the purple colour of octahedral [Ti(H2O)6]3+
ion by d-d electron transition
• [Ti(H2O)6]3+ is an octahedral complex ion in which Ti-
atom is present as Ti3+ ion whose valence-shell electronic
configuration is 3d1.
• According to crystal field theory when six water molecules (which
act as ligands) approach the central Ti3+ ion to form the octahedral
complex ion, [Ti(H2O)6]3+, the five d-orbitals of Ti3+ ion split into
t2g (dxy, dyz and dzx) and eg (dx
2
-y
2 and dz
2) sets of orbitals.
• This phenomenon is called crystal field splitting.
• t2g set of orbitals has lower energy than eg set of orbitals and hence
d1 electron of Ti3+ ion resides in t2g set and eg set remains vacant.](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-15-320.jpg)
![• Thus the ground state electronic configuration of Ti3+ ion
can be written as t1
2g e0
g.
• Now when white light is allowed to fail on [Ti(H2O)6]3+
ion, this ion absorbs green radiation.
• Since the ion absorbs green radiation, the colour of the
transmitted radiation would be white light minus green
light (colour of the absorbed light) which is purple.
• Thus [Ti(H2O)6]3+ ion looks purple to our eyes.
• The absorption of green light by [Ti(H2O)6]3+ ion takes
place at a wavelength of about 5000Å as is evident from
the visible absorption spectrum which has been obtained
by plotting a graph between the wavelength (in Å) of the
absorbed light and the amount of absorbed light
(absorbance) (see following figure).](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-16-320.jpg)
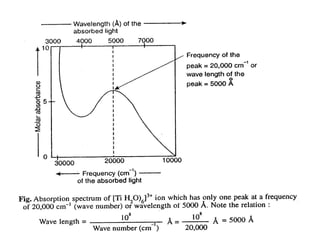
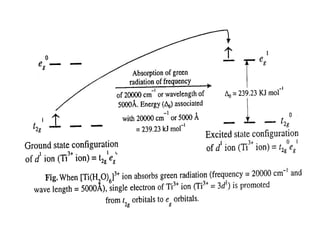
![• 5000Å is the wavelength of the band of maximum
absorption.
• Obviously the energy (Δo) associated with the wavelength
of 5000Å.
• This energy (= 239 kJ mol-1) is close to the crystal field
splitting energy, Δo (also called excitation energy)
(Excitation energy is the energy difference between t2g
and eg orbitals) and, therefore, the electron present in t2g
orbitals absorbs 239 kJ mol-1 and is excited to occupy the
vacant eg orbitals (t1
2g → e0
g electron transition) so that
the electronic configuration of Ti3+ ion in the excited state
becomes t0
2ge1
g (see following figure).
• Thus we see that the purple colour of [Ti(H2O)6]3+ ion is due to the
excitation of an electron from the d-orbitals of lower energy (t2g
orbitals) to the d-orbitals of higher energy (eg orbitals).](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-18-320.jpg)
![• We have already said that the magnitude of Δo (Δo is the
energy difference between t2g and eg orbitals) is inversely
proportional to that of the wavelength of the absorbed
radiation.
• This means that a complex ion having higher value of Δo will
absorb the radiation of lower wavelength and vice versa.
Evidences
• This fact is evident from the following examples of octahedral
complex ions of Co3+ and Ni2+ ions:
a) Since the value of Δo for [Co(CN)6]3- ion is higher than that of
[Co(NH3)6]3+ (see following table).
• [Co(CN)6]3- ion absorbs violet radiation which has lower
wavelength.
Higher Δo → lower λ
Higher Δo for [Co(CN)6]3- → lower Δo for [Co(NH3)6]3+
[Co(CN)6]3- → violet → lower λ → yellow green](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-20-320.jpg)
![• On the other hand [Co(NH3)6]3+ ion absorbs blue green radiation
which has higher wavelength.
• Thus [Co(CN)6]3- ion appears yellow green in solution and
[Co(NH3)6]3+ ion appears orange or pink in solution.
b) Since [Ni(H2O)6]2+ ion has lower value of Δo than [Ni(NH3)6]2+ ion,
[Ni(NH3)6]2+ ion absorbs yellow colour which has lower
wavelength while [Ni(H2O)6]2+ ion absorbs red colour which has
higher wavelength.
• Thus [Ni(NH3)6]2+ ion in solution has blue colour but [Ni(H2O)6]2+
ion in solution has blue green colour.
[Co(NH3)6]3+ → blue green → higher λ → orange or pink
[Ni(NH3)6]2+
Ni2+ ion → Yellow → Blue
[Ni(H2O)6]2+
Ni2+ ion → Red → Blue green
[Ni(NH3)6]2+ → Yellow → λ 5750 - 5900 Å→ Δo 10800 cm-1→ Blue
[Ni(H2O)6]2+ → Red → λ 6250 - 7500 Å → Δo 8500 cm-1 → Blue green](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-21-320.jpg)
![B) Colour of the complex ions whose central atom contains empty
or completely-filled d-orbitals
• The transition metal complex ions whose central atom contains
empty (d0 configuration) or completely-filled d-orbitals (d10
configuration) are colourless.
• This is because of the fact that since the d-orbitals of the central
metal ion donot contain any electron (empty d-orbitals) or are
completely-filled (i.e., contain all the electrons in the paired state),
d-d electron transition is not possible and hence no light of any
colour or wavelength is absorbed by such ions in the visible region.
• Thus the transition metal ions containing:
– Empty d-orbitals [e.g., Sc3+ and Ti4+ (TiO2) ions)] or
– Completely-filled d-orbitals [e.g., Cu+, Ag+, Zn2+ (ZnSO4), Cd2+,
Hg2+ etc.]
are colourless.](https://image.slidesharecdn.com/colourofmetalcomplexes-201221090508/85/Colour-of-metal-complexes-24-320.jpg)