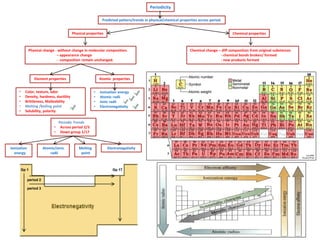
The document summarizes key concepts about the periodic table including periods, groups, and blocks. It explains that periods are horizontal rows based on the same principal quantum number, while groups are vertical columns with the same number of valence electrons. Blocks refer to different regions (s, p, d, f) based on which orbitals are being filled with electrons. It provides examples of trends in ionization energy and atomic radius across periods and down groups.

![s block elements
• s orbitals partially fill
1
H
He
p block elements
• p orbital partially fill
5
1s2
n = 2 period 2
B
[He] 2s2 2p1
6
1s1
2
Periodic Table – s, p d, f blocks elements
C
[He] 2s2 2p2
7
N
[He] 2s2 2p3
3
Li
[He] 2s1
8
O
[He] 2s2 2p4
4
Be
[He] 2s2
9
F
[He] 2s2 2p5
11
Na
[Ne] 3s1
10
Ne
[He] 2s2 2p6
12
Mg
[Ne] 3s2
13
Al
[Ne] 3s2 3p1
14
20
K
Ca
[Ne] 3s2 3p2
[Ar]
15
P
[Ne] 3s2 3p3
[Ar]
4s2
16
S
[Ne] 3s2 3p4
17
19
Si
4s1
CI
[Ne] 3s2 3p5
18
Ar
[Ne] 3s2 3p6
d block elements
• d orbitals partially fill
• transition elements
21
Sc
[Ar] 4s2 3d1
22
Ti
[Ar] 4s2 3d2
23
V
[Ar] 4s2 3d13
24
Cr
[Ar] 4s1 3d5
25
Mn
[Ar] 4s2 3d5
26
Fe
[Ar] 4s2 3d6
27
Co
[Ar] 4s2 3d7
28
Ni
[Ar] 4s2 3d8
29
Cu
[Ar] 4s1 3d10
30
Zn
[Ar] 4s2 3d10
f block elements
• f orbitals partially fill
Video on electron configuration
Click here electron structure
Click here video on s,p,d,f notation
Click here video s,p,d,f blocks,](https://image.slidesharecdn.com/periodictrend-140226025644-phpapp01/85/IB-Chemistry-on-Periodic-Trends-Effective-Nuclear-Charge-and-Physical-properties-2-320.jpg)


![IE drop from Mg to AI and P to S
Ionization Energy- Period 3
Why IE increases across the period 3?
IE increases across period 3
Nuclear charge increase
Strong electrostatic forces
attraction bet nucleus and e
IE – High
Na
Mg
AI
Si
P
S
CI
Ar
3p
3s
[Ne] 3s1
[Ne] 3s2
[Ne] 3s2 3p1
[Ne] 3s2 3p2
IE drop from Mg to AI
[Ne] 3s2 3p3
[Ne] 3s2 3p4
IE drop from P to S
Electron in p sublevel of AI
– further away from nucleus
2 electrons in same p orbital
- Greater e/e repulsion
Weak electrostatic force attraction
between nucleus and electron
Easier to remove e
IE - Low
IE - Low
Period 3
[Ne] 3s2 3p5
[Ne] 3s2 3p6](https://image.slidesharecdn.com/periodictrend-140226025644-phpapp01/85/IB-Chemistry-on-Periodic-Trends-Effective-Nuclear-Charge-and-Physical-properties-6-320.jpg)
![IE for Period 2 and 3
Ionization Energy- Period 2 and 3
Why IE period 3 lower than 2?
Period 3 – 3 shells/energy level
Period 3
Valence e further from nucleus
High shielding effect – more inner e
Weaker electrostatic forces
attraction bet nucleus and e
IE – Lower
period 2
Li
Be
B
C
N
O
F
Ne
2p
2s
1s
1s2 2s1
1s2 2s2
1s2 2s2 2p1
1s2 2s2 2p2
1s2 2s2 2p3
1s2 2s2 2p4
1s2 2s2 2p5
1s2 2s2 2p6
Period 3
Na
Mg
AI
Si
P
S
[Ne] 3s2 3p1
[Ne] 3s2 3p2
[Ne] 3s2 3p3
[Ne] 3s2 3p4
CI
Ar
3rd level
3p
3s
2p
2s
1s
[Ne] 3s1
[Ne] 3s2
[Ne] 3s2 3p5
[Ne] 3s2 3p6](https://image.slidesharecdn.com/periodictrend-140226025644-phpapp01/85/IB-Chemistry-on-Periodic-Trends-Effective-Nuclear-Charge-and-Physical-properties-7-320.jpg)
![IE for Ne and Ar
Ionization Energy- Period 2 and 3
Why Ne and Ar have HIGH IE ?
Full electron configuration, 2.8/2.8.8
neon
argon
Most energetically stable structure
Difficult to lose electron
IE – High
period 2
Li
Be
B
C
N
O
F
Ne
2p
2s
1s
1s2 2s1
1s2 2s2
1s2 2s2 2p1
1s2 2s2 2p2
1s2 2s2 2p3
1s2 2s2 2p4
1s2 2s2 2p5
1s2 2s2 2p6
Period 3
Na
Mg
AI
Si
P
S
[Ne] 3s2 3p1
[Ne] 3s2 3p2
[Ne] 3s2 3p3
[Ne] 3s2 3p4
CI
Ar
3p
3s
2p
2s
1s
[Ne] 3s1
[Ne] 3s2
[Ne] 3s2 3p5
[Ne] 3s2 3p6](https://image.slidesharecdn.com/periodictrend-140226025644-phpapp01/85/IB-Chemistry-on-Periodic-Trends-Effective-Nuclear-Charge-and-Physical-properties-8-320.jpg)








