1. The document discusses the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts molecular geometry based on electron pair repulsion.
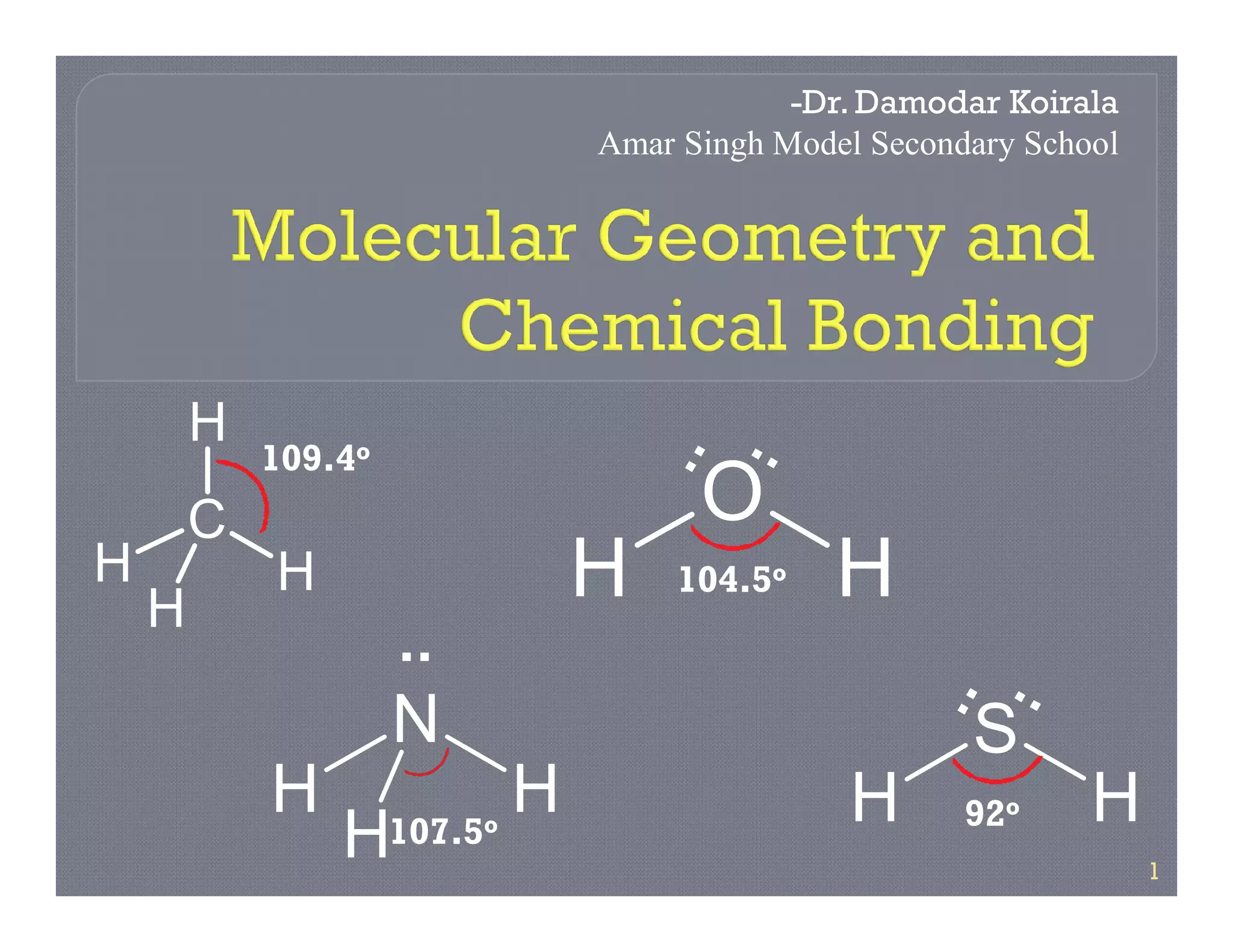
2. VSEPR theory states that electron pairs around a central atom arrange themselves as far apart as possible to minimize repulsion. This determines if a molecule is linear, trigonal planar, tetrahedral, etc.
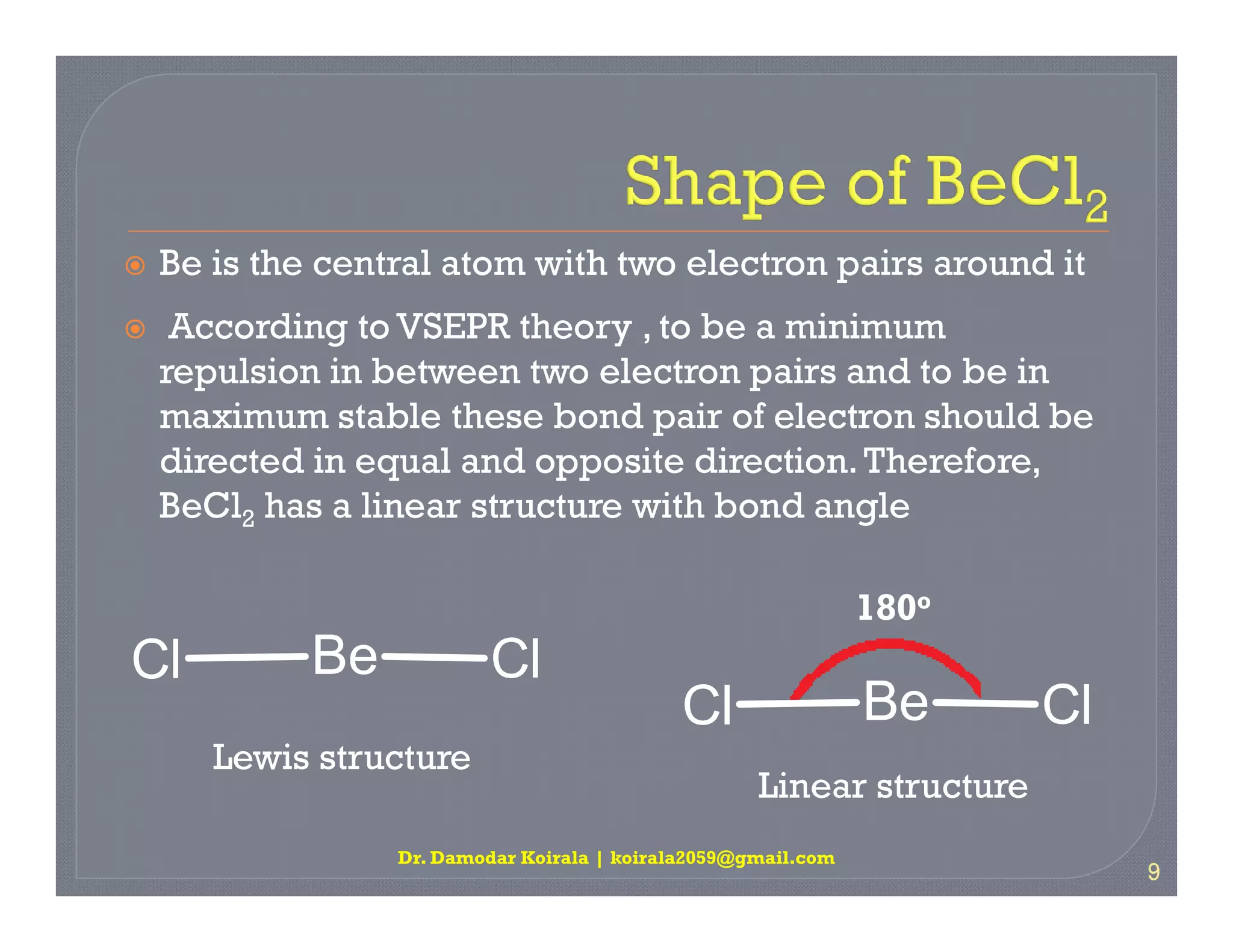
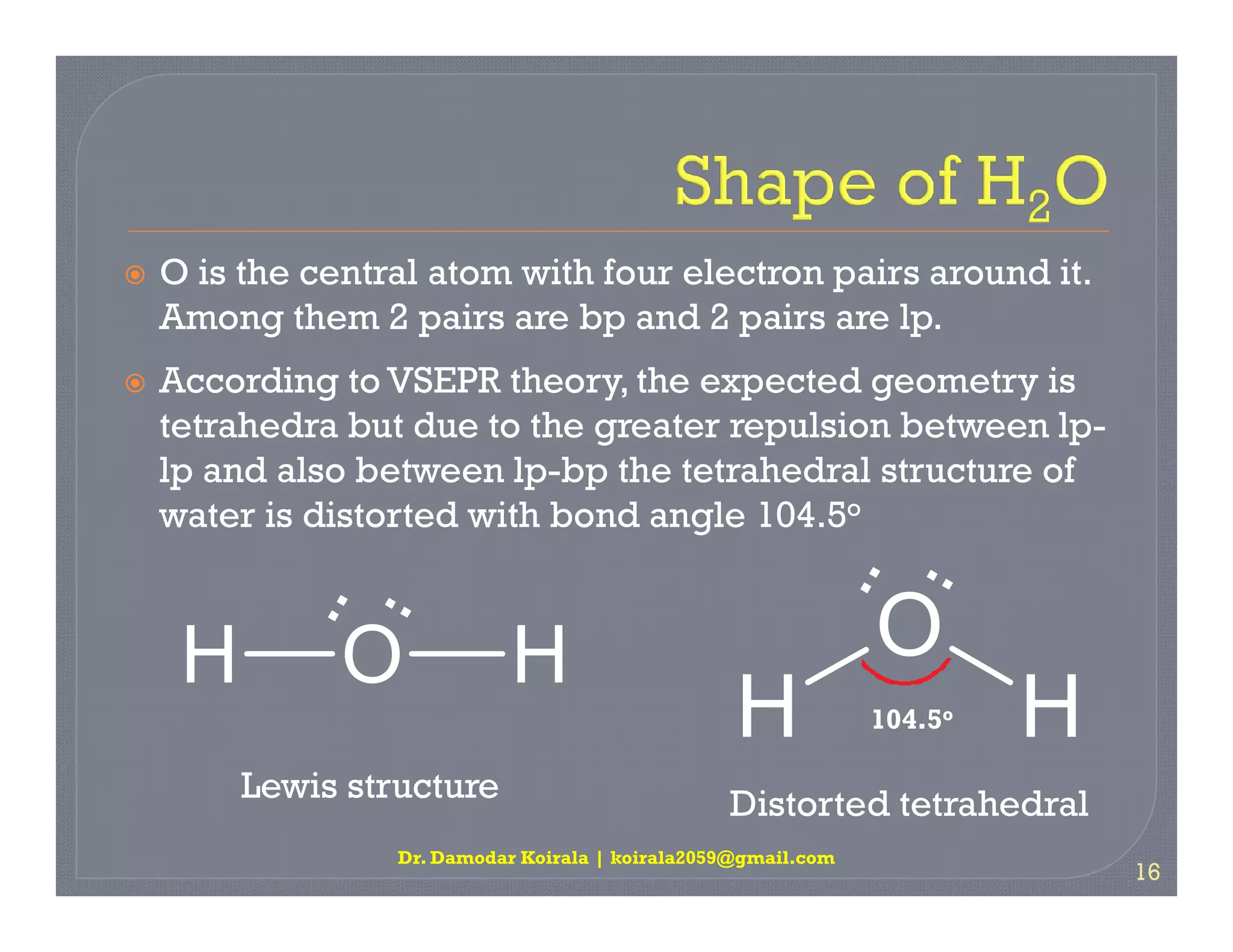
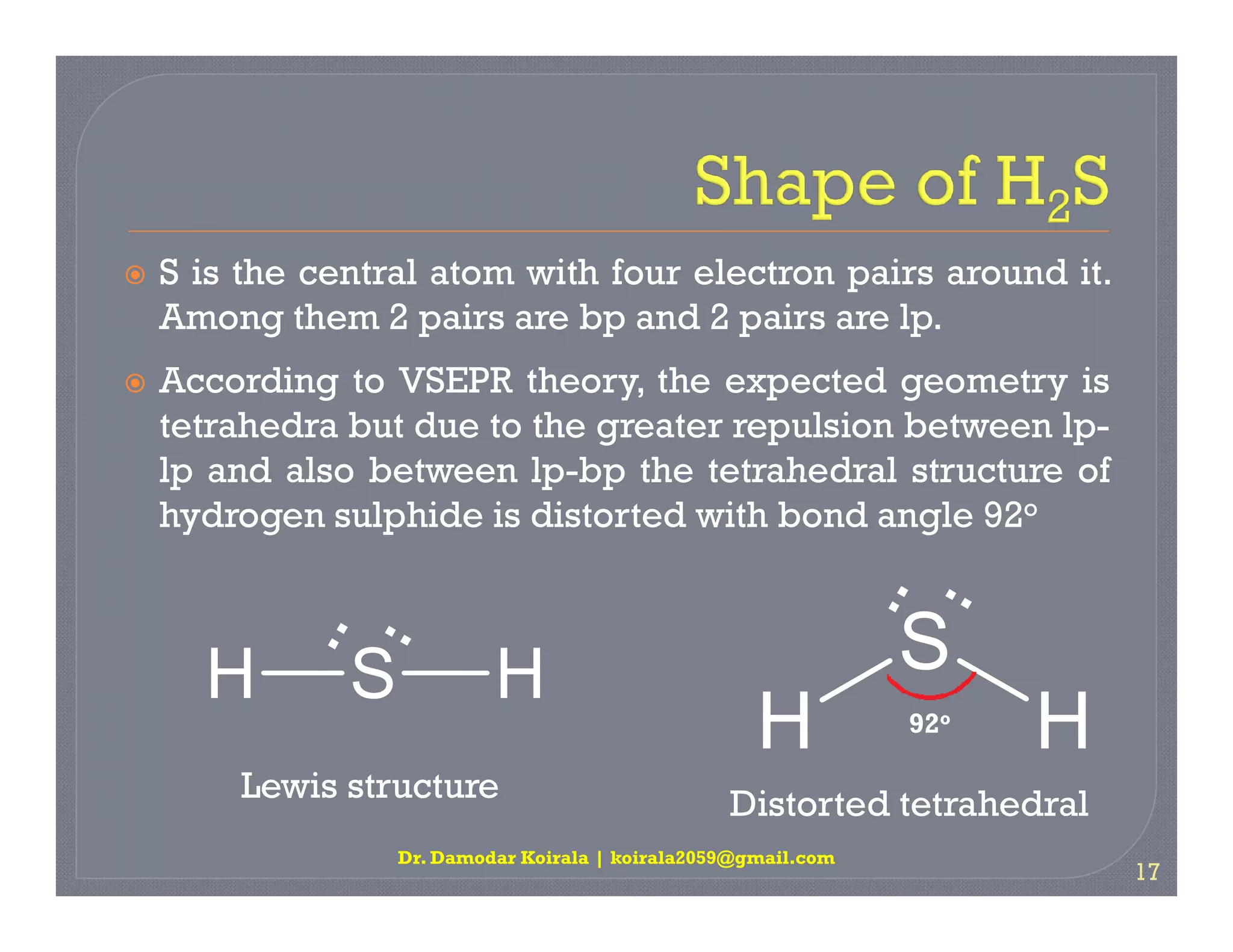
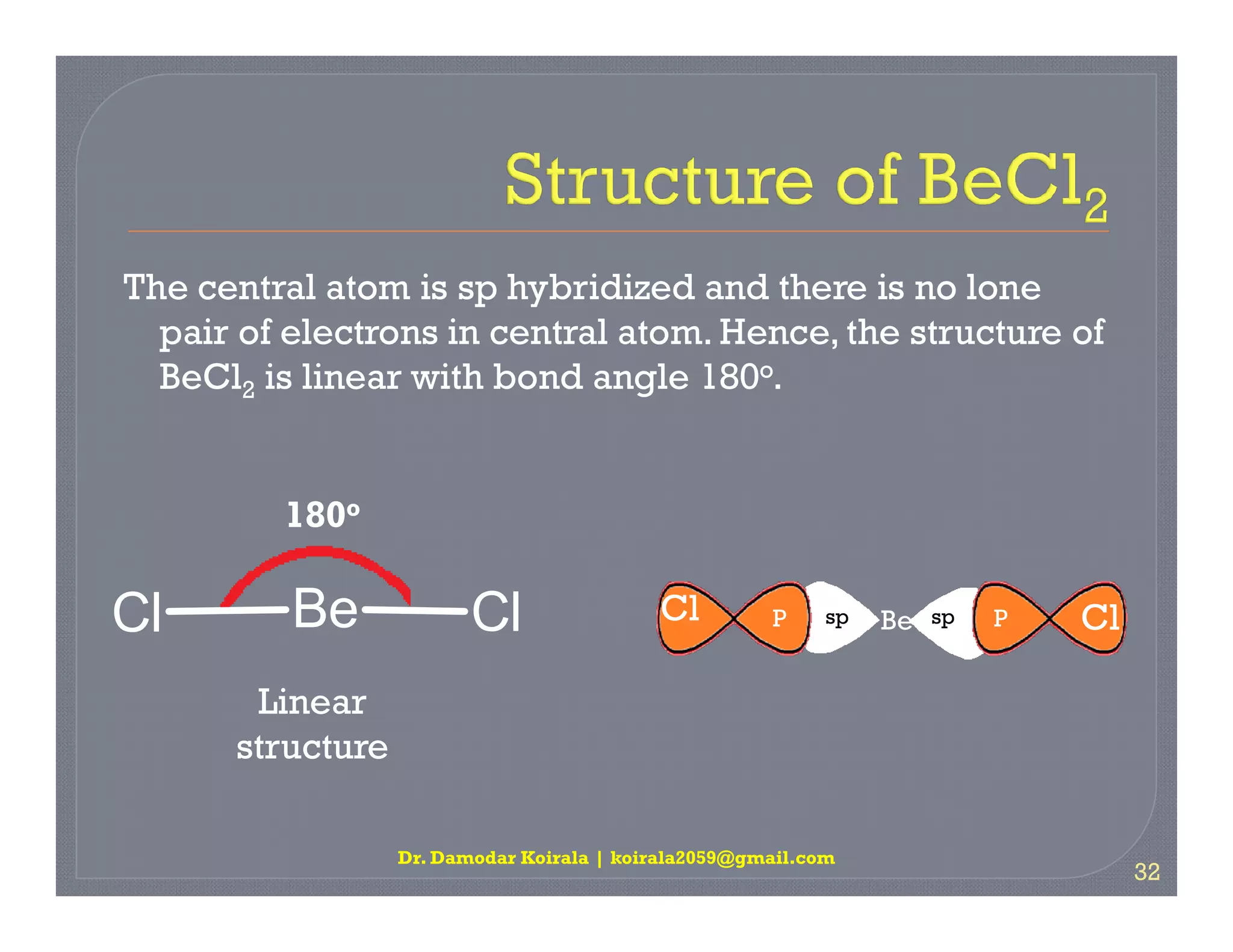
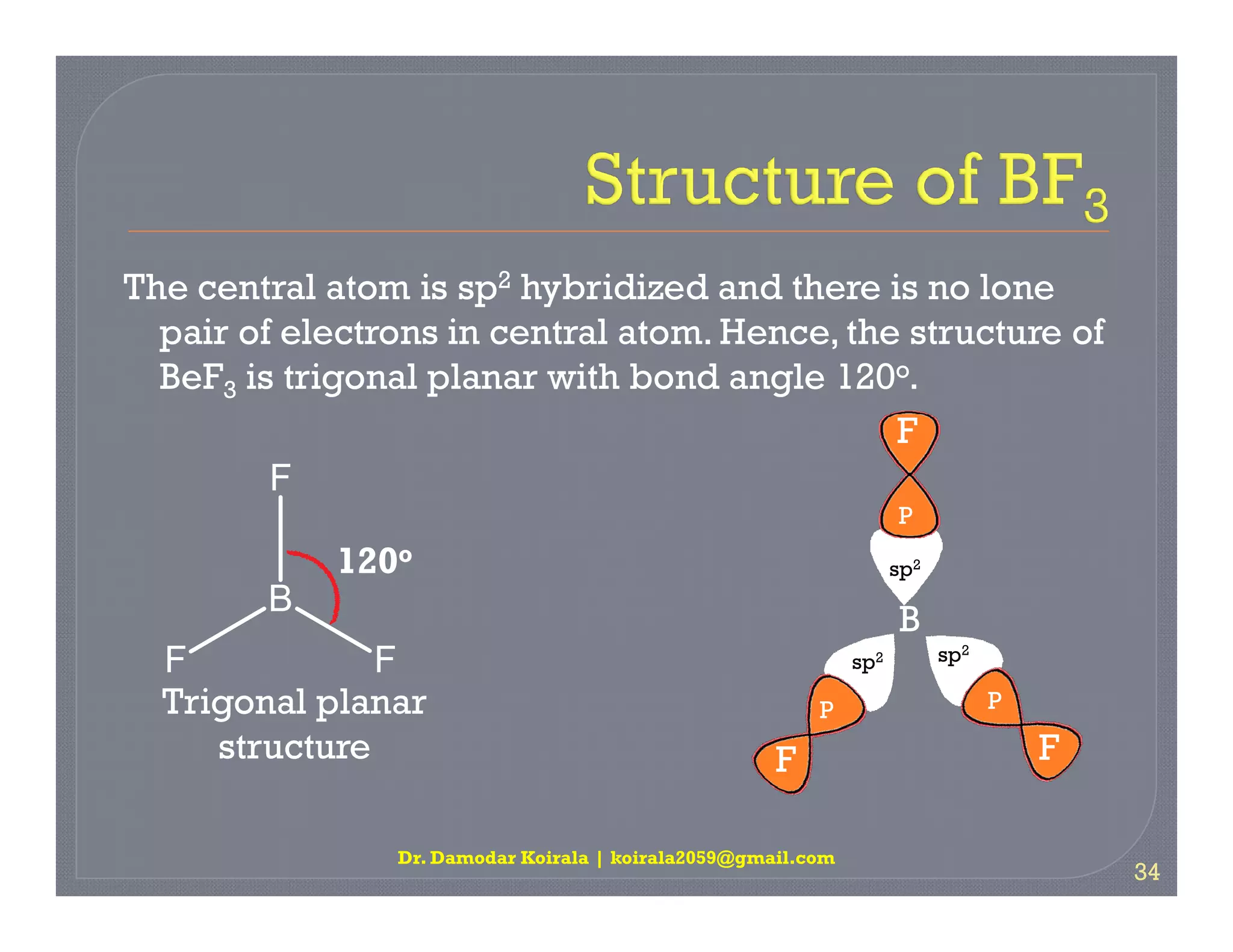
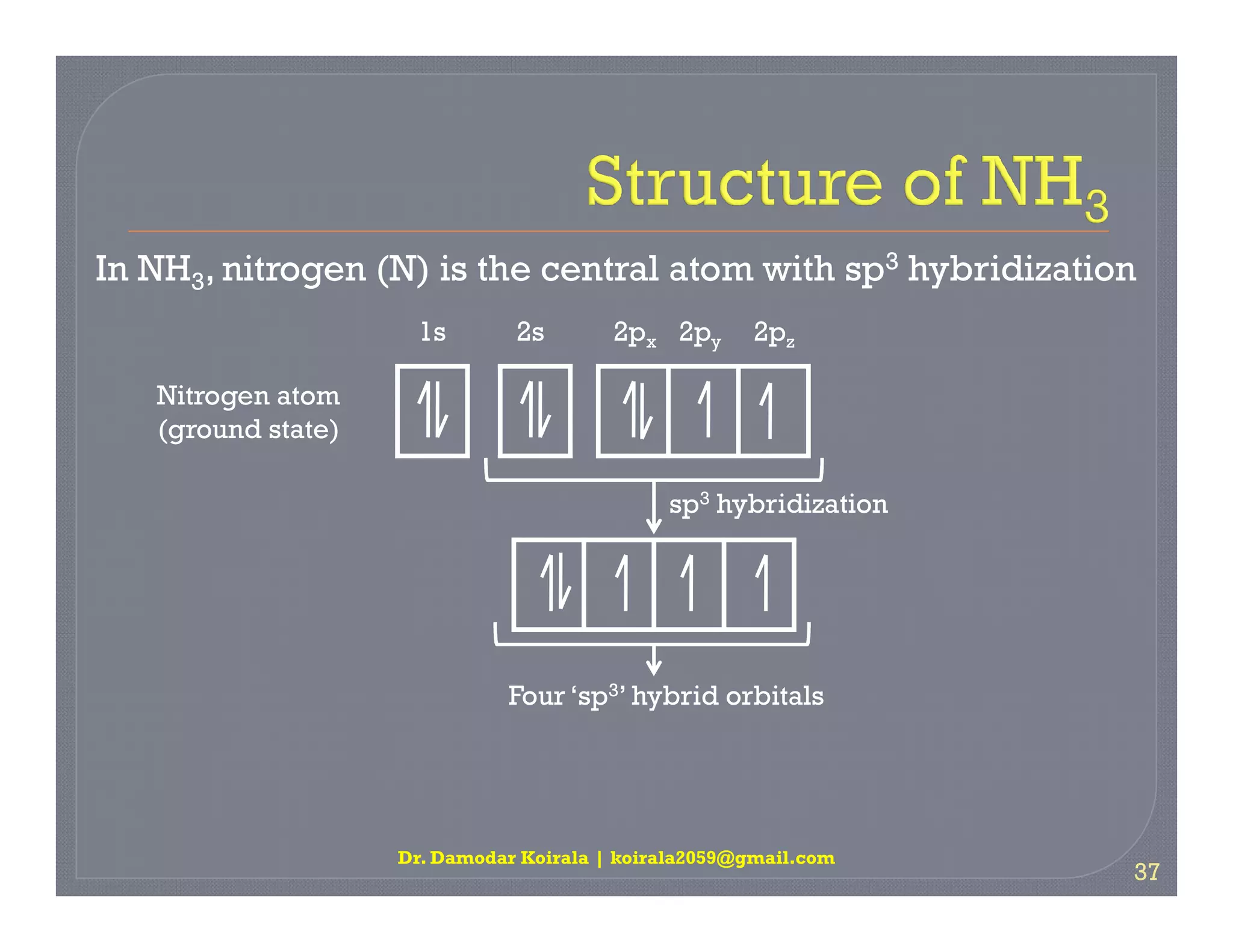
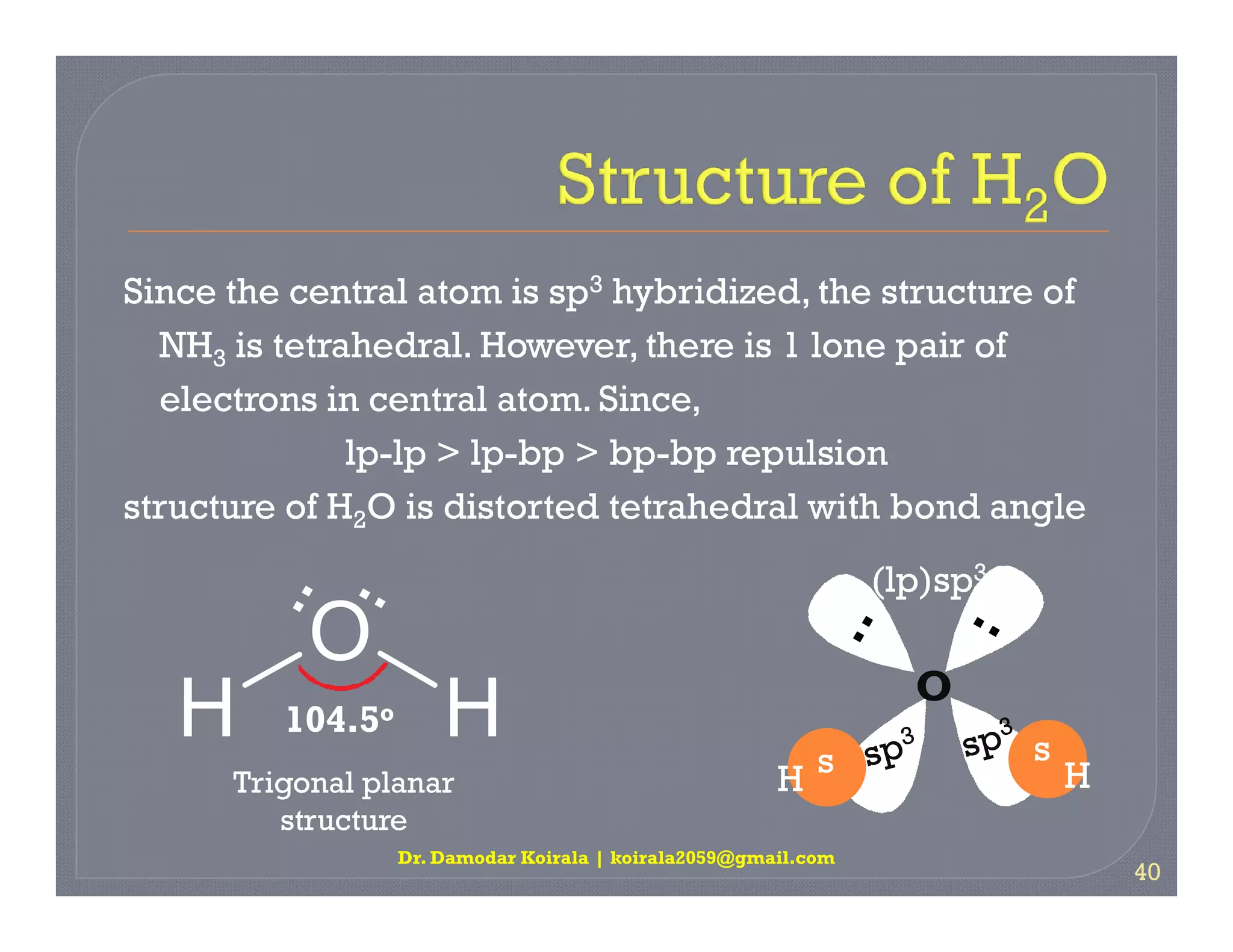
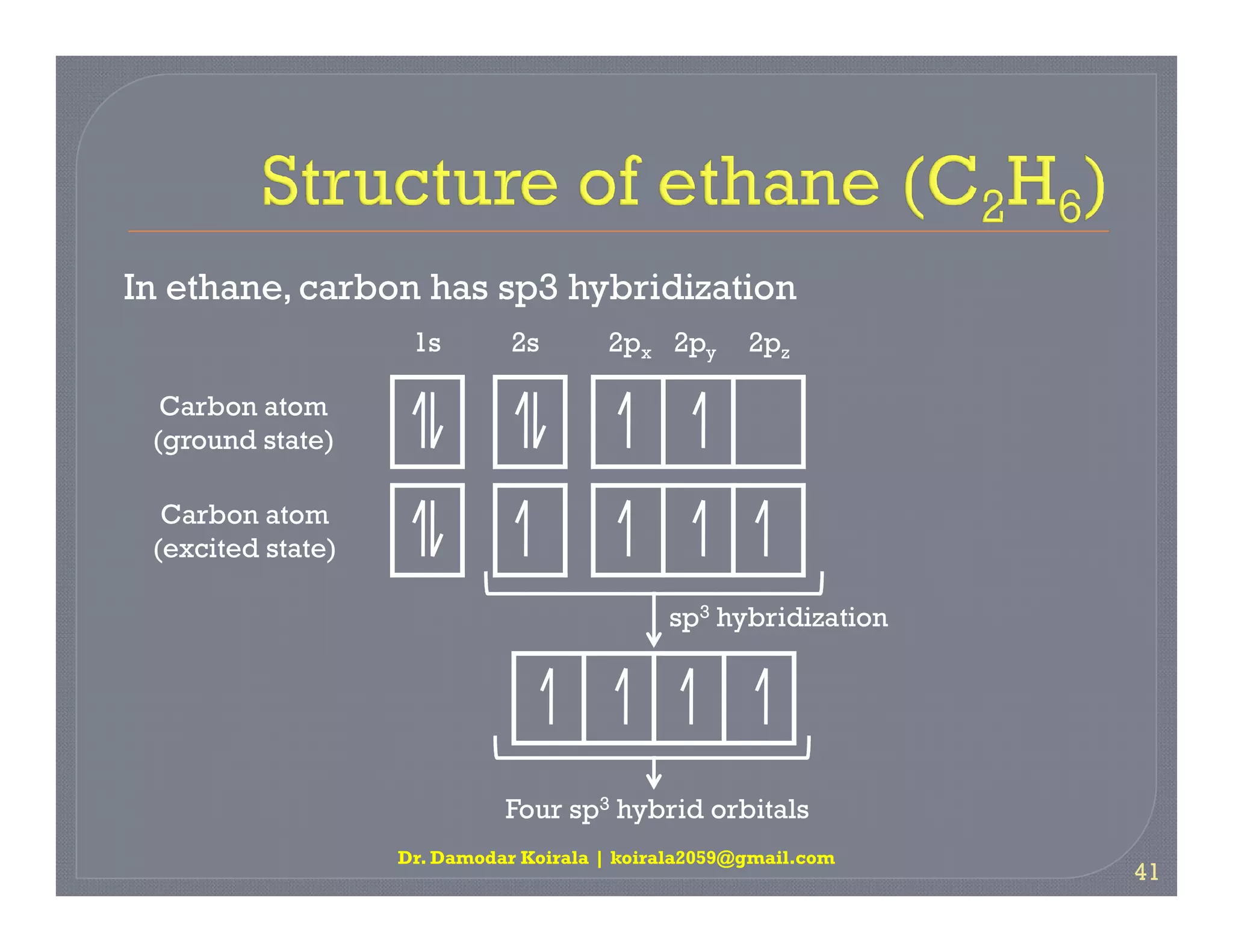
3. The document provides examples of applying VSEPR theory to determine the structures of various molecules such as BeCl2, BF3, CH4, NH3, H2O. Lone electron pairs can cause distortions from regular geometries.