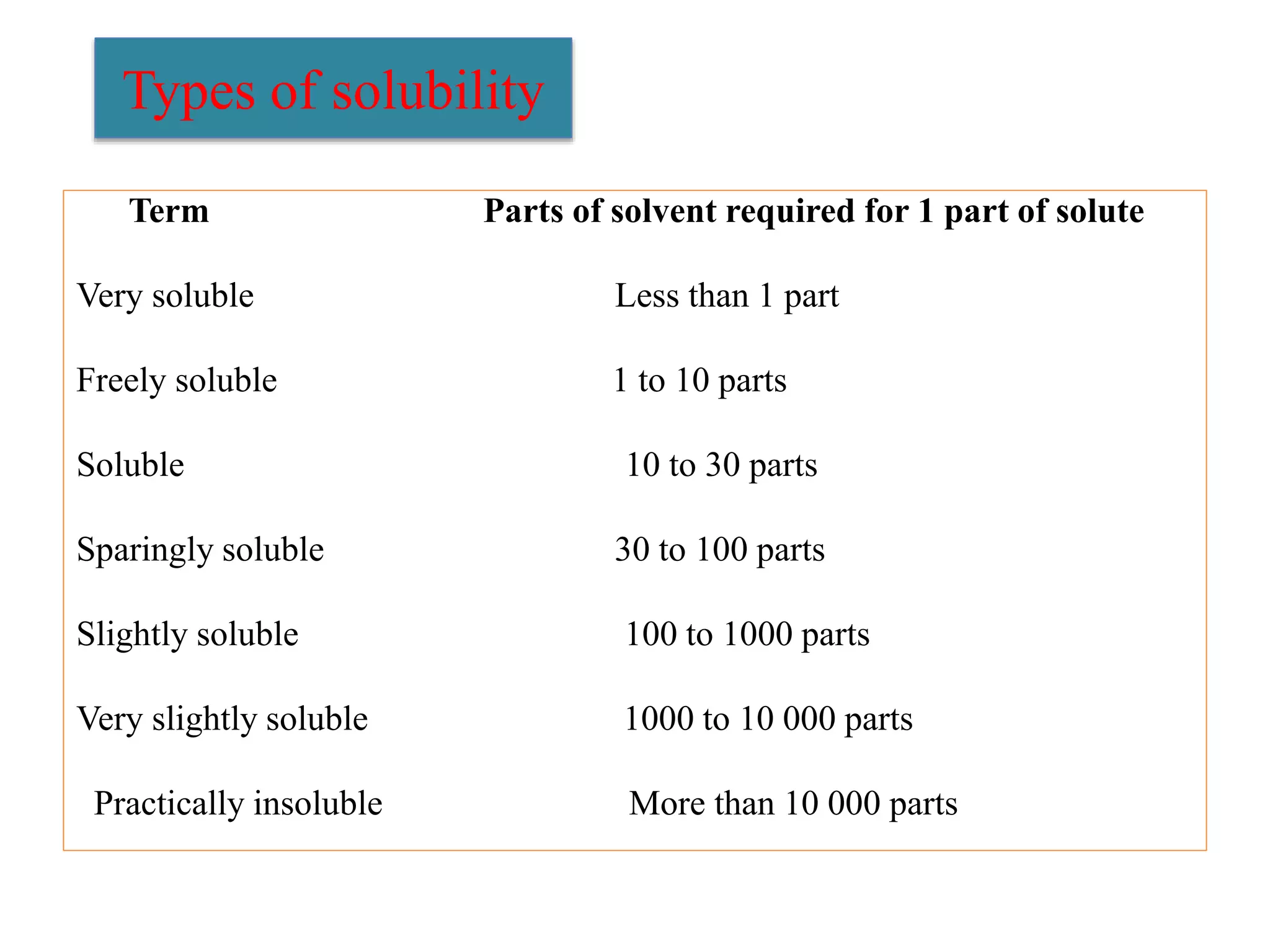
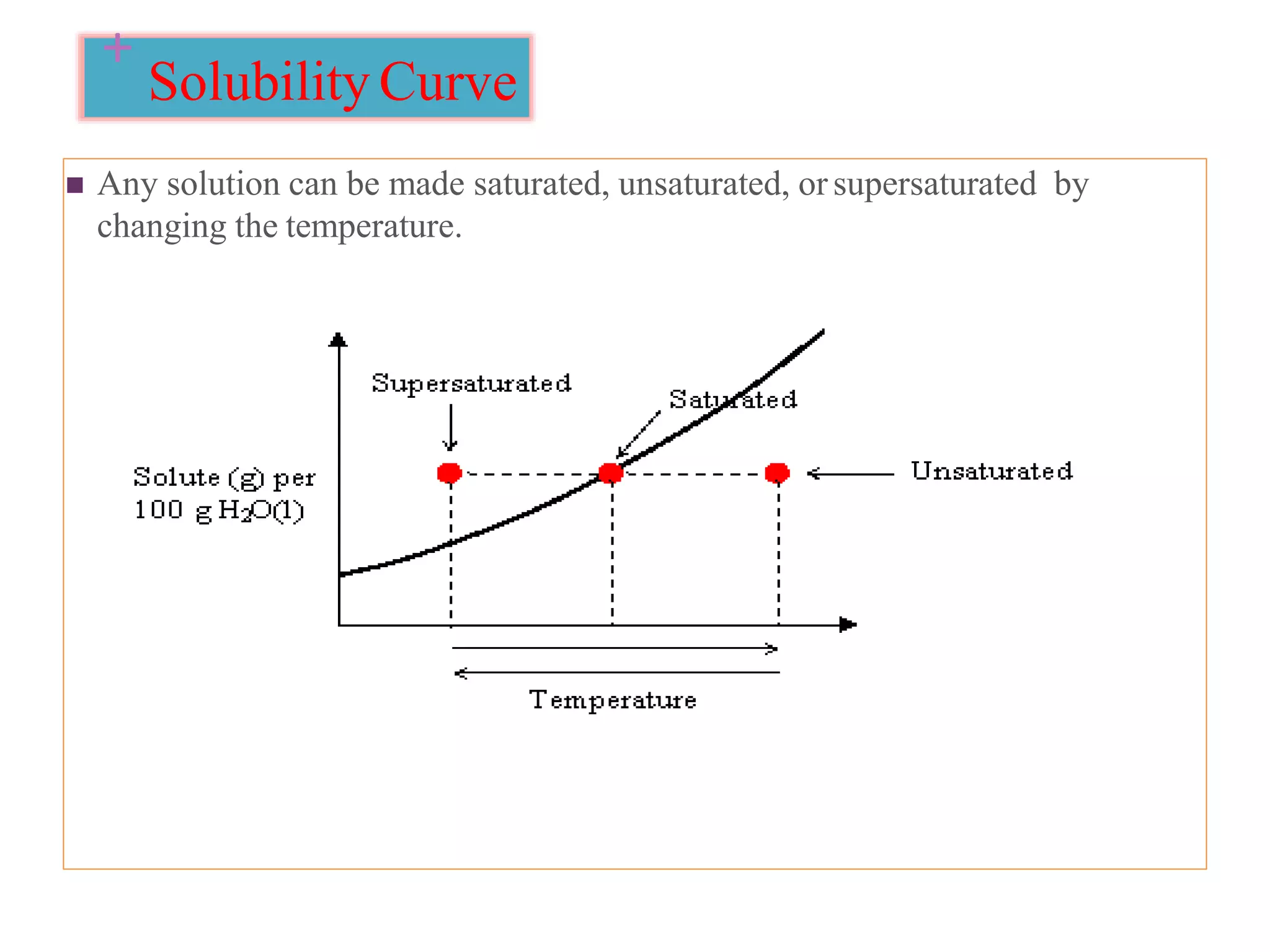
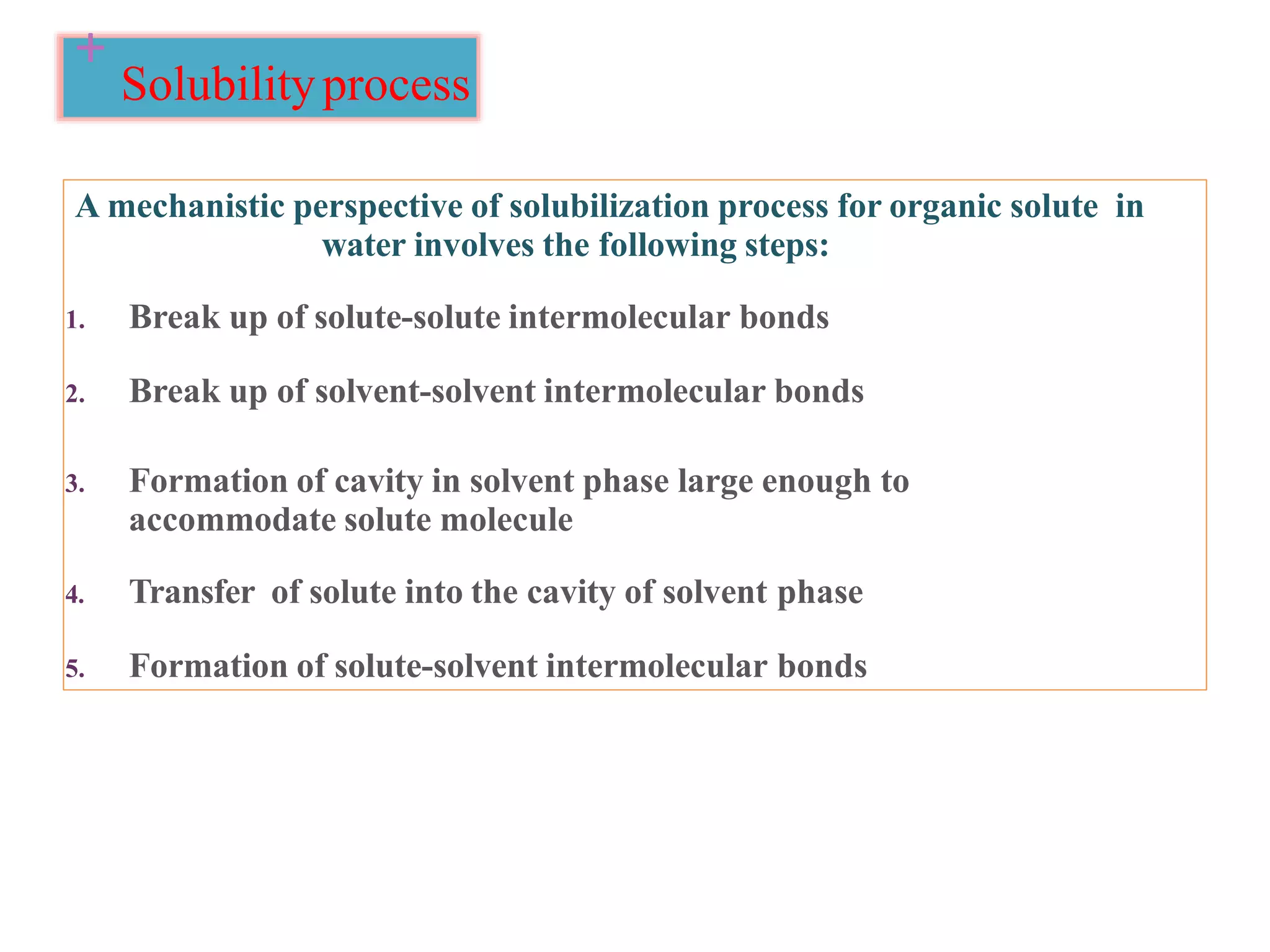
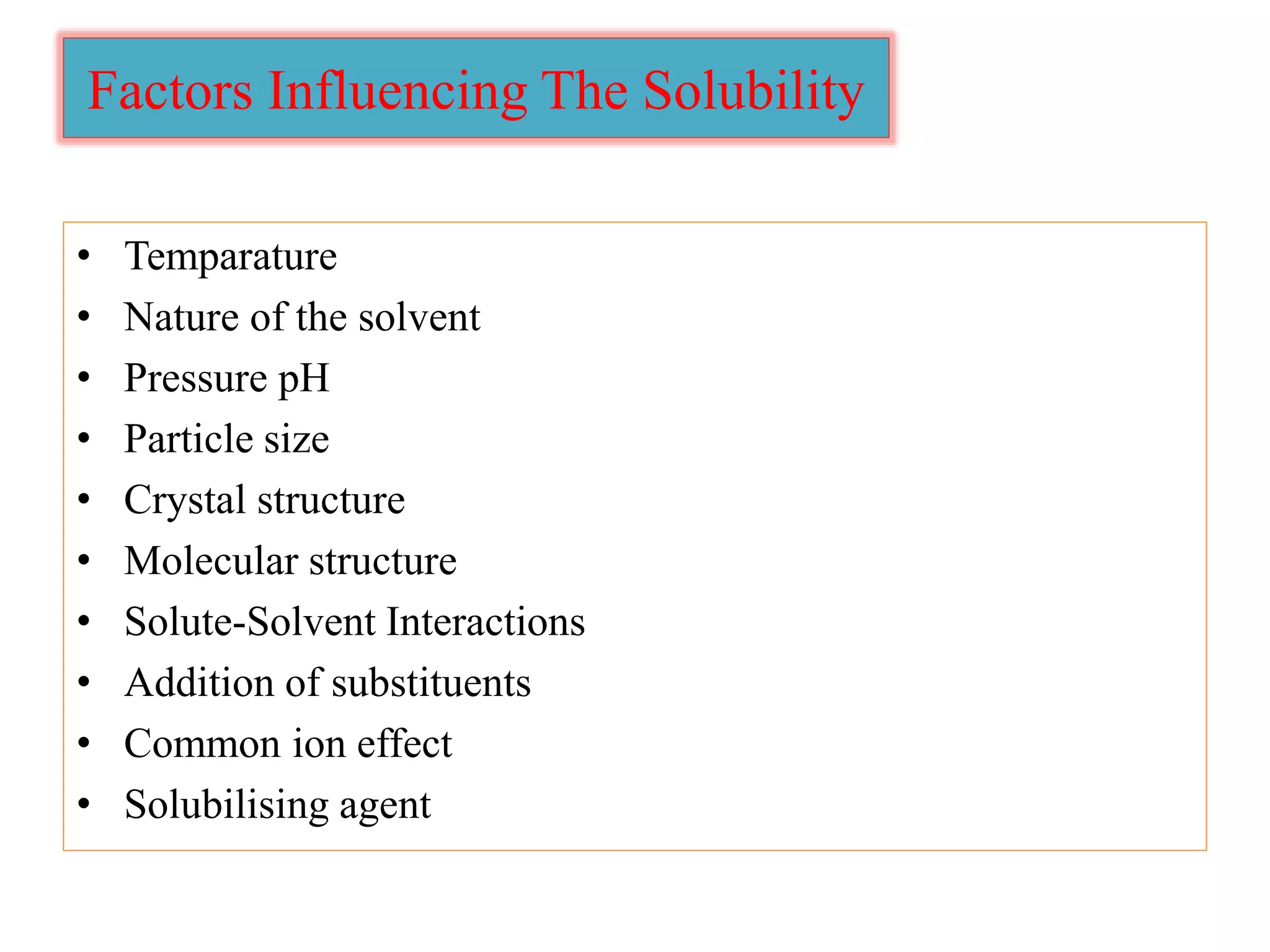
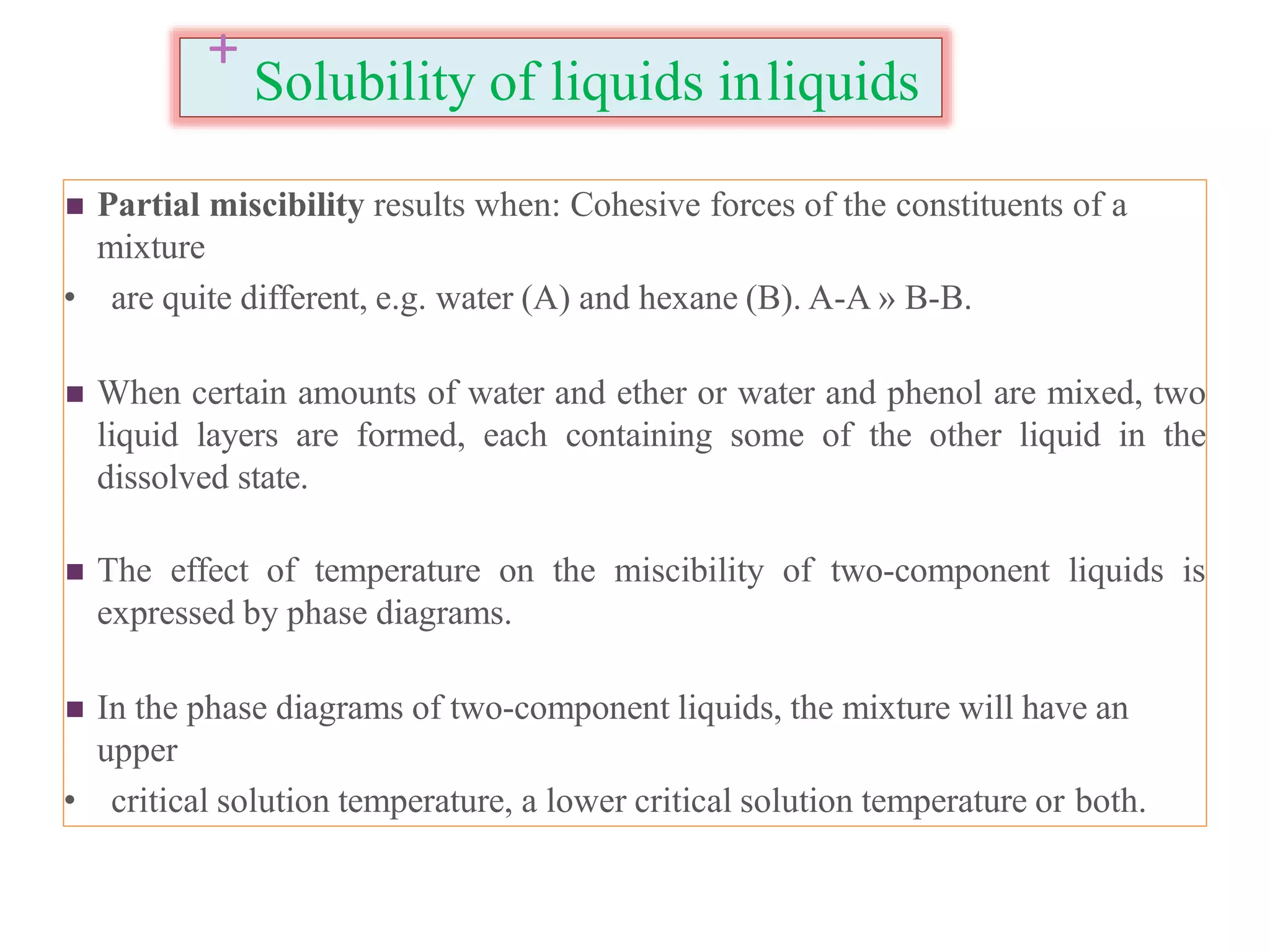
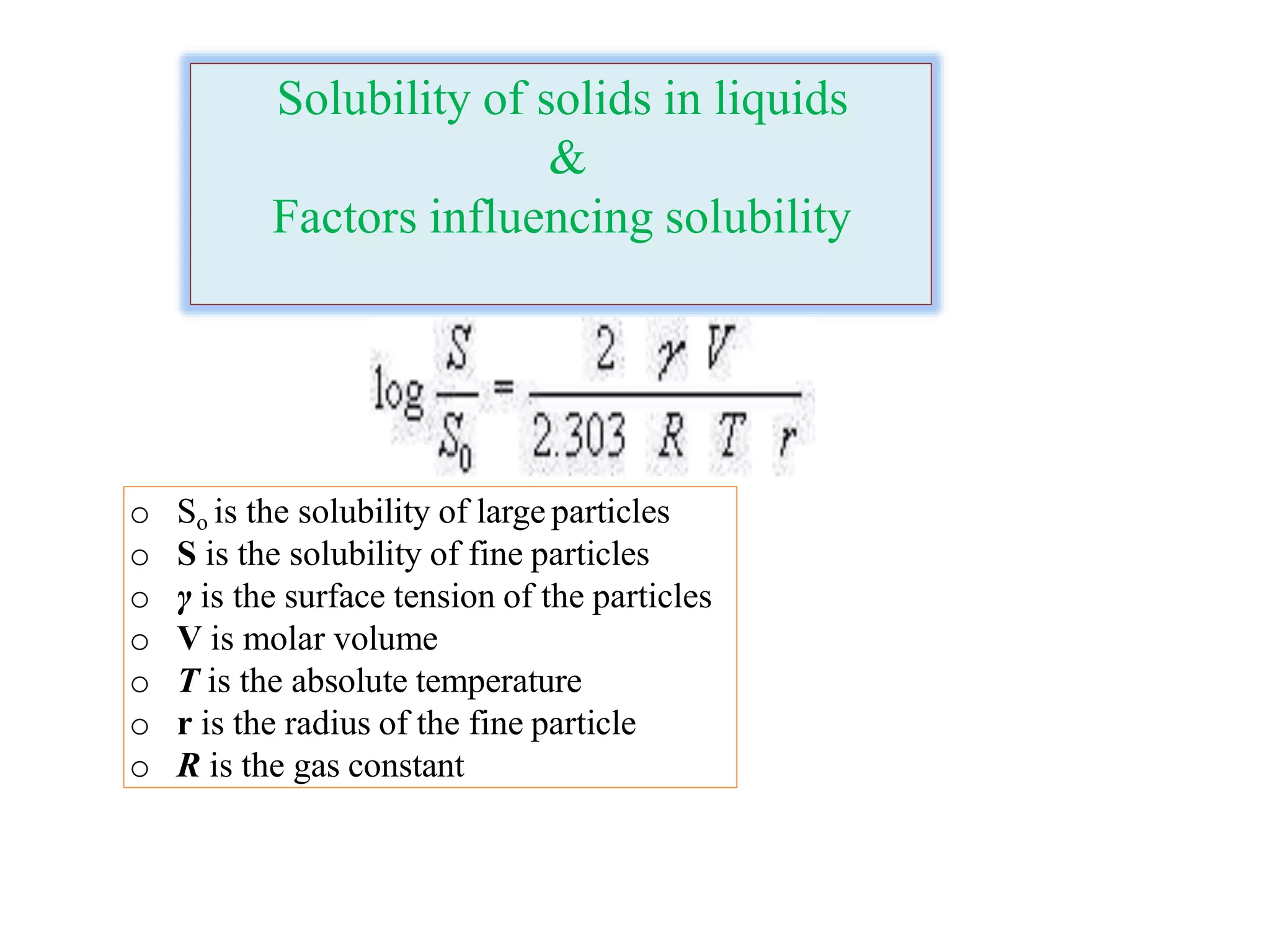
The document discusses the concept of solubility, detailing its definition, expression, types, and influencing factors. It emphasizes the importance of understanding solubility for pharmacists in selecting solvents and formulating solutions, highlighting interactions between solute and solvent. Additionally, it covers various solvent classifications and their mechanisms, as well as the influence of parameters like temperature, pH, and molecular structure on solubility.