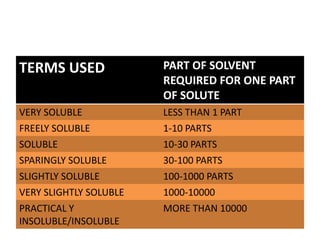
This document discusses solubility, including definitions, expressions, mechanisms, factors affecting solubility, and approaches to enhancing solubility. Solubility refers to the property of a solid, liquid, or gas dissolving in a solid, liquid, or gas. It can be expressed quantitatively based on parts of solvent required to dissolve one part of solute. Solubility depends on factors like temperature, pressure, nature of solute and solvent, and can be enhanced through approaches like reducing particle size, modifying crystal habit, using surfactants or complexation, chemical modification of solute, or co-crystallization.