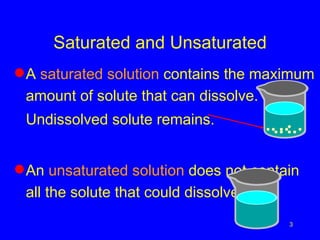
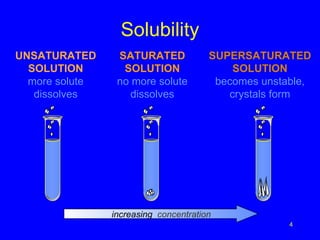
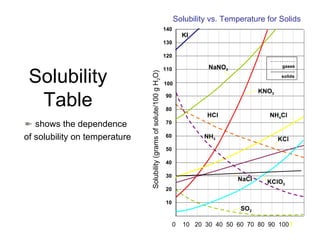
Solubility refers to the maximum amount of solute that can dissolve in a solvent, usually 100g. A saturated solution contains the maximum amount of solute, while an unsaturated solution does not. The solubility of most solids increases with temperature, while the solubility of most gases decreases with temperature. High temperatures can cause carbonated drinks to burst or fish to die due to decreased gas solubility in water.