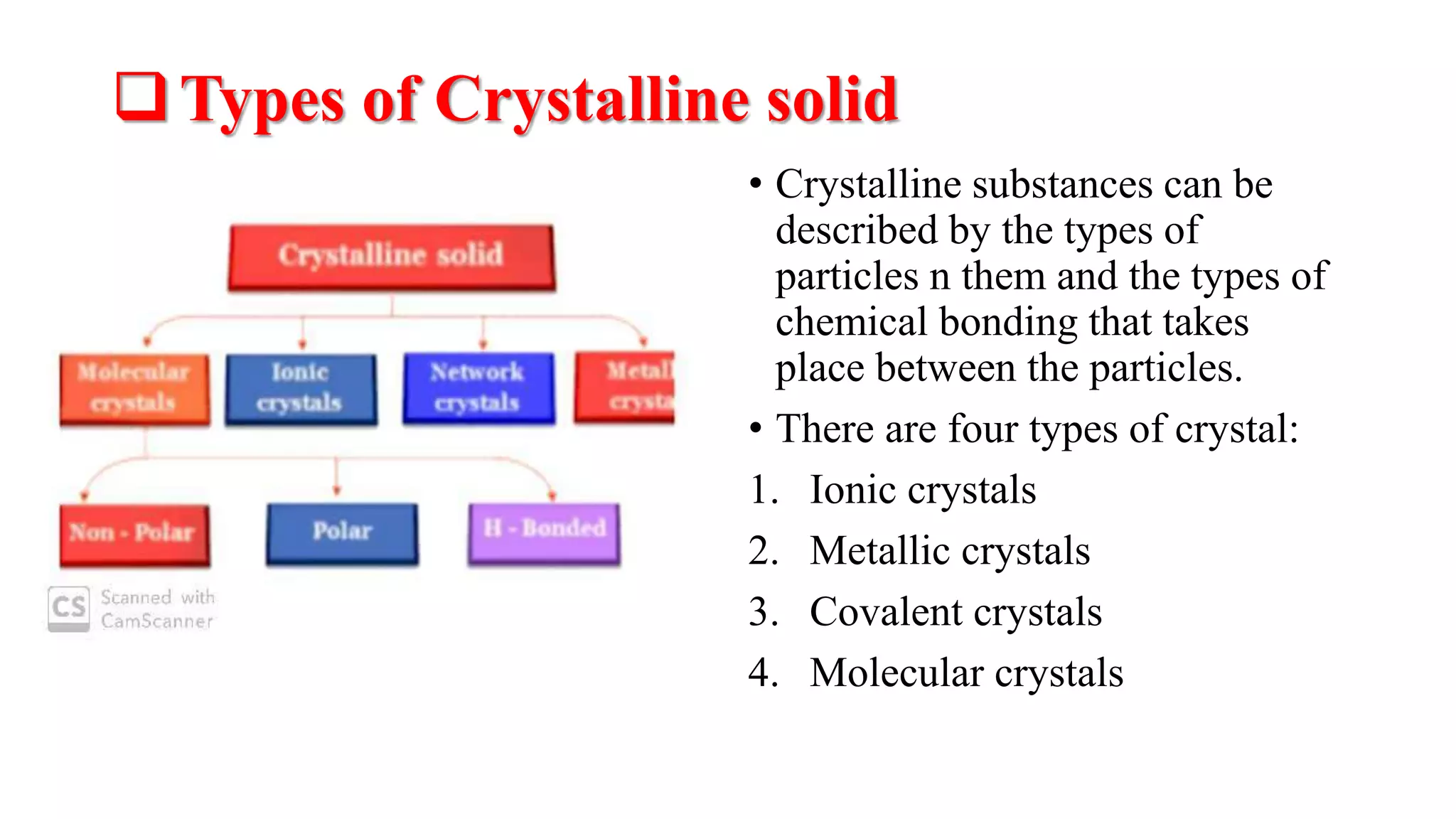
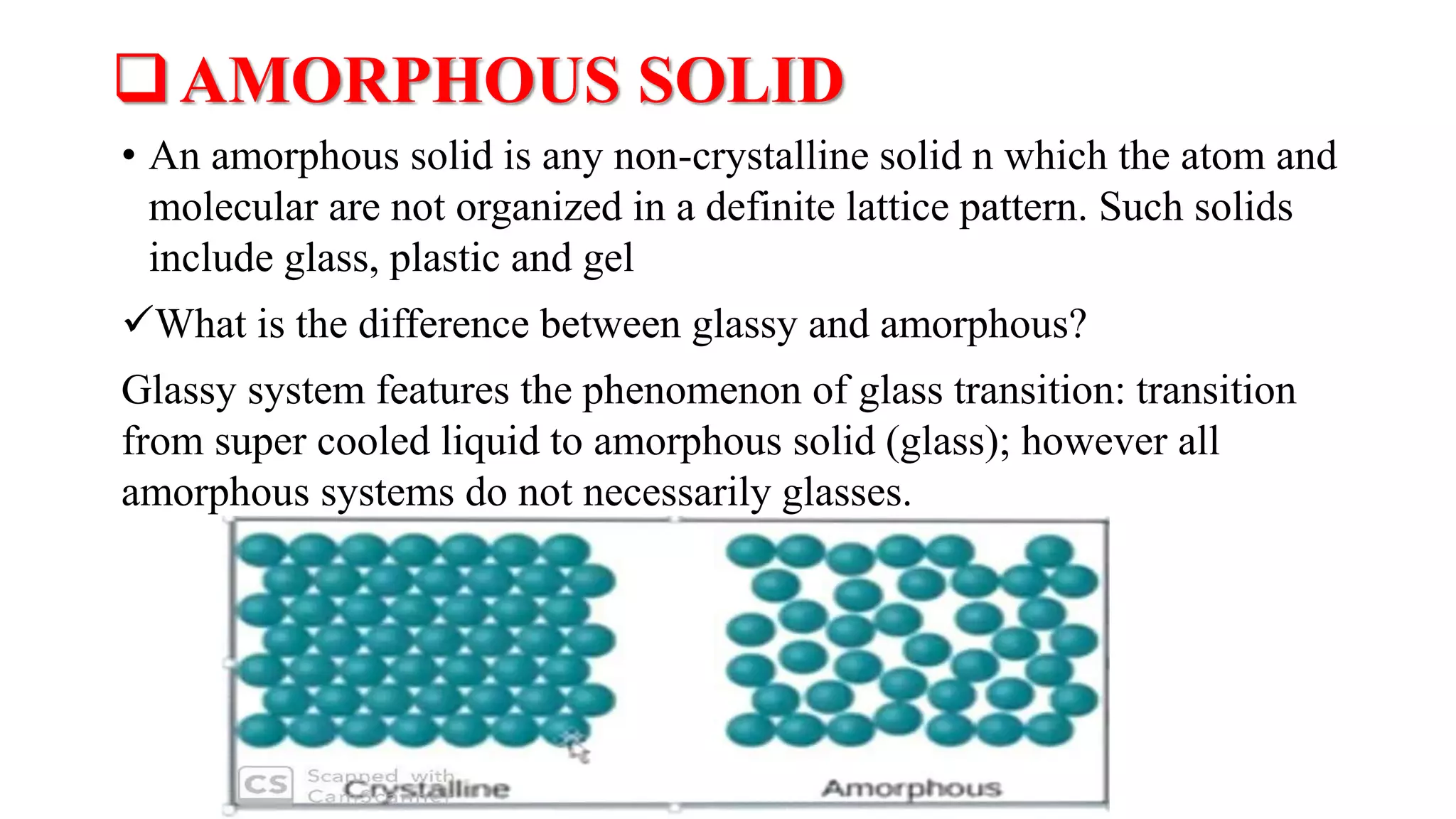
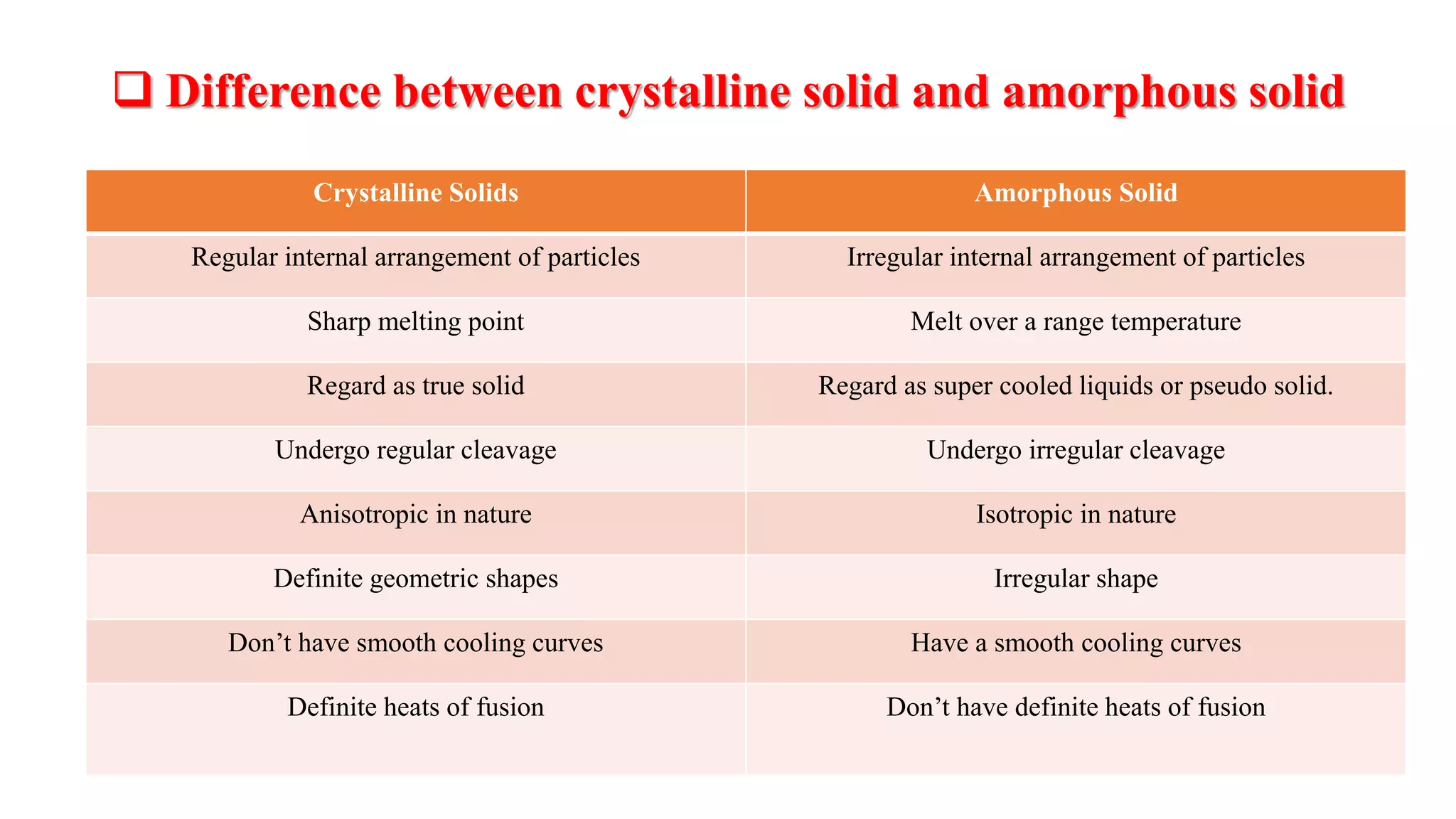
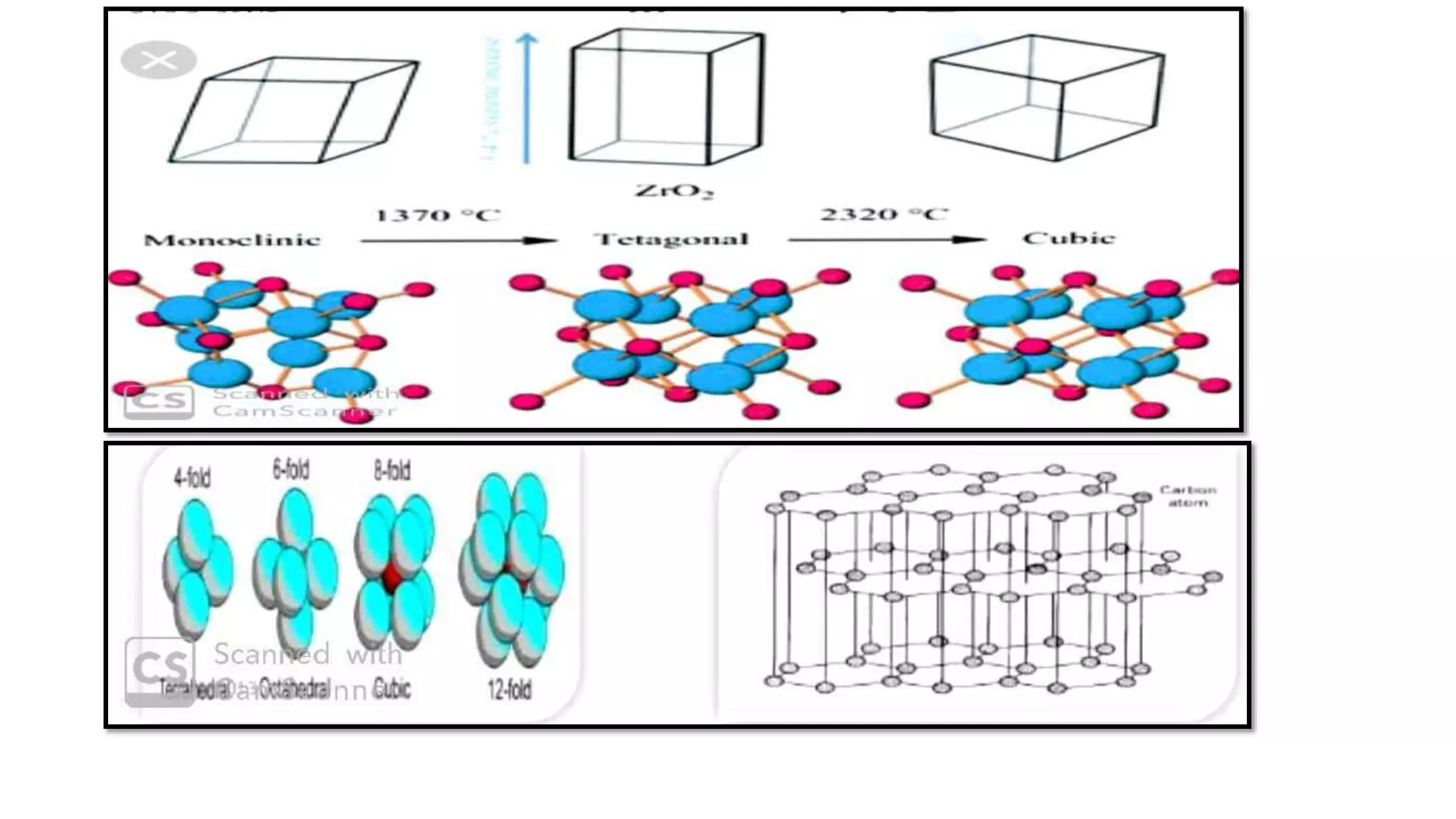
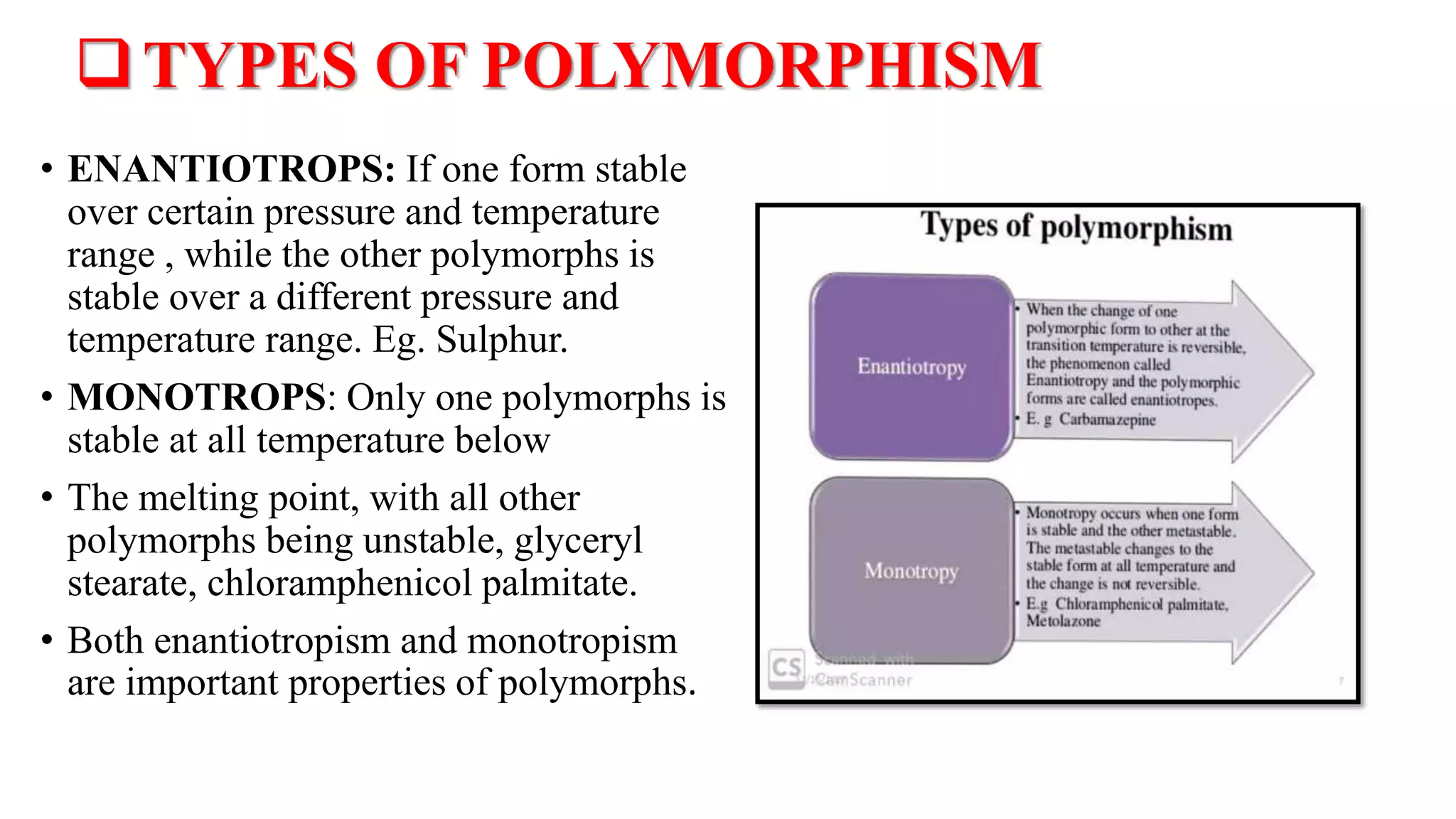
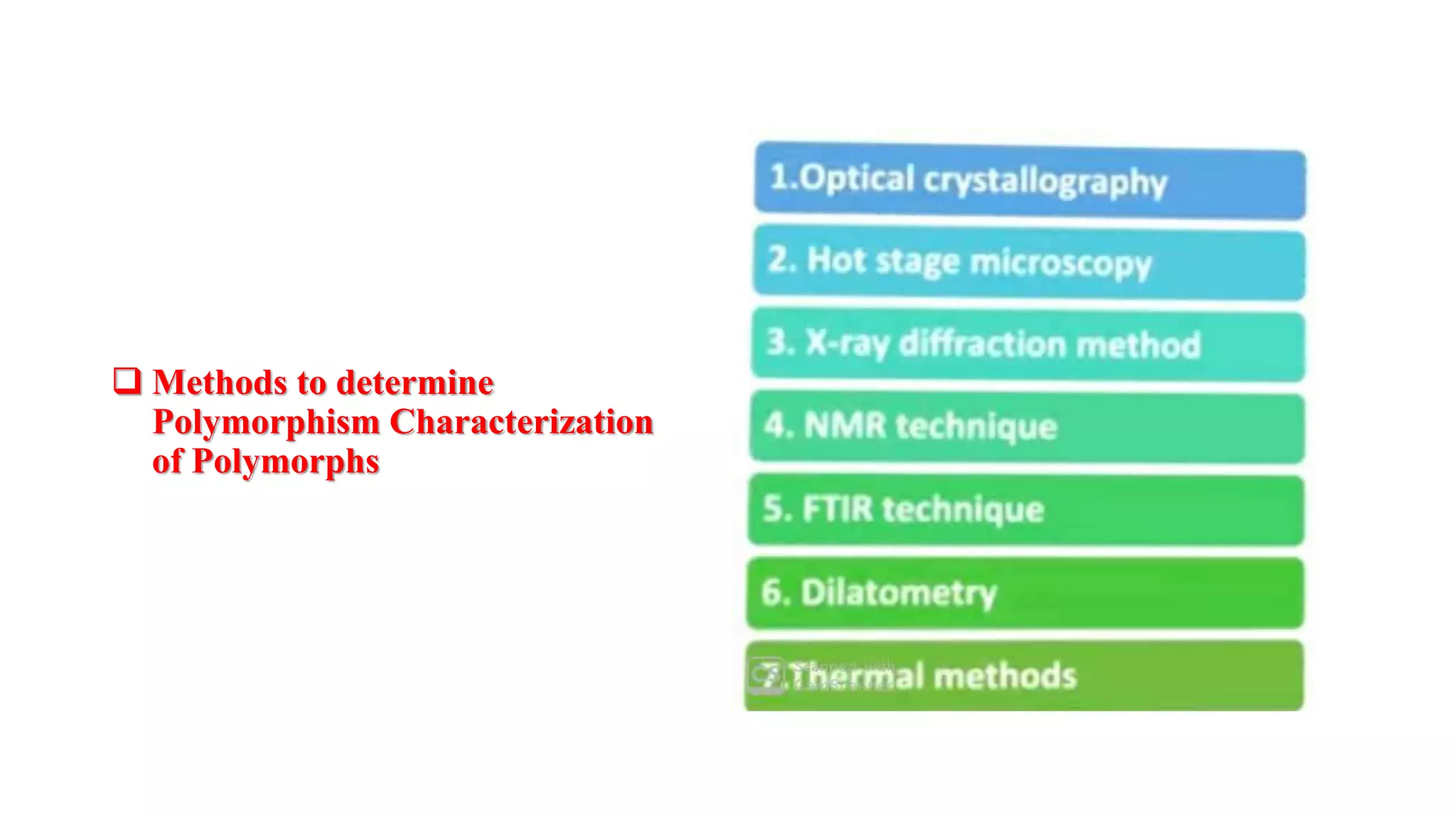
The document provides an overview of the state and properties of matter, focusing on crystalline and amorphous solids, as well as polymorphism. Crystalline solids have an ordered atomic arrangement, while amorphous solids lack this structure, affecting their properties and bioavailability. Additionally, the document discusses polymorphism, including its types, properties, and significance in pharmaceutical applications.