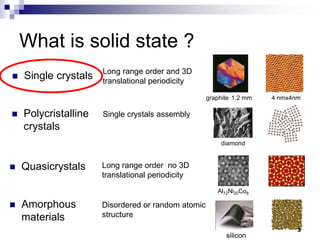
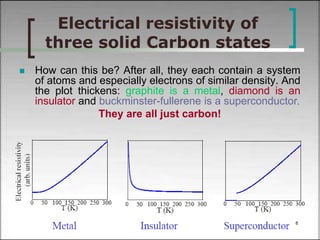
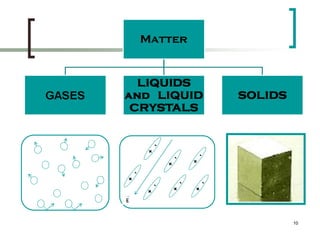
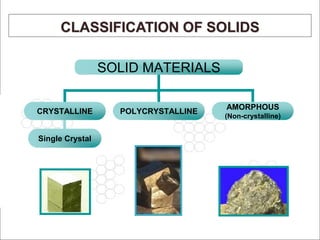
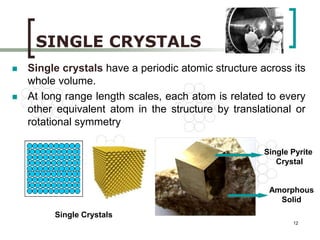
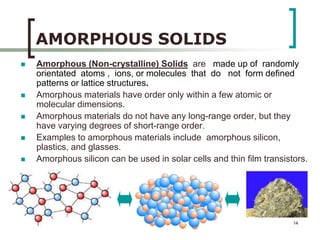
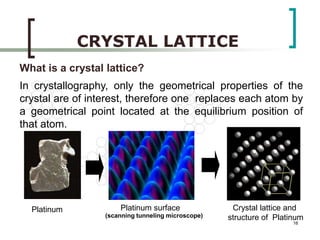
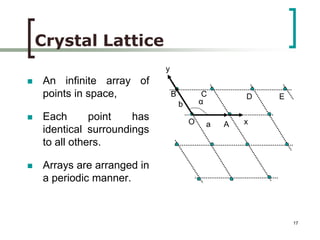
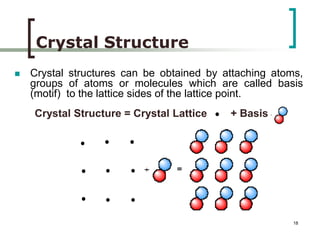
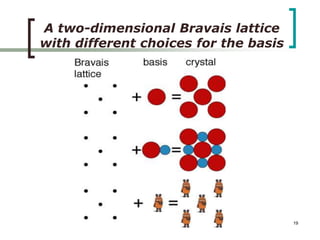
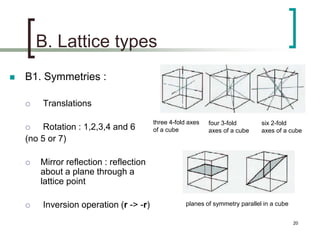
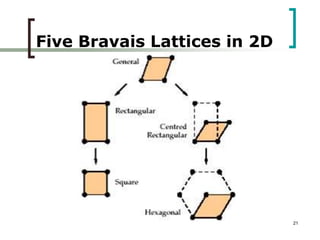
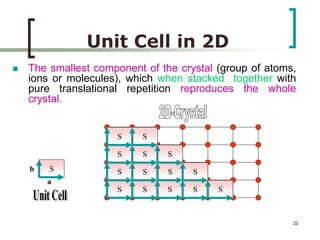
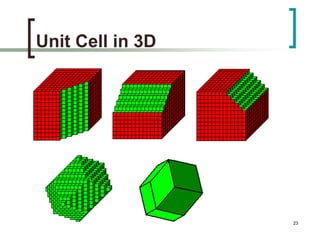
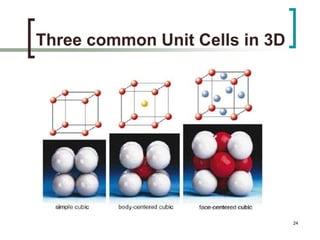
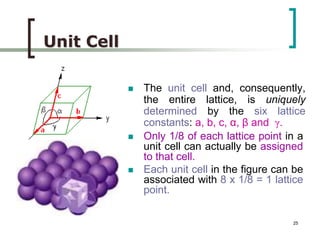
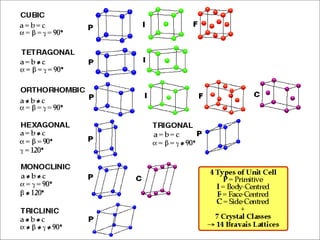
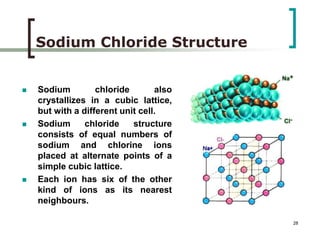
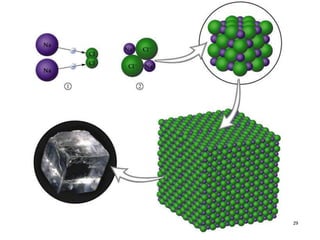
This document discusses solid state physics and crystal structures. It begins by defining solid state physics as explaining the properties of solid materials by analyzing the interactions between atomic nuclei and electrons. It then discusses different types of solids including single crystals, polycrystalline materials, and amorphous solids. Single crystals have long-range periodic atomic order, while polycrystalline materials are made of many small crystals joined together and amorphous solids lack long-range order. The document goes on to describe crystal structures including crystal lattices, unit cells, and common crystal systems such as cubic, hexagonal, and orthorhombic. It provides examples of crystal structures including sodium chloride and its cubic lattice structure.