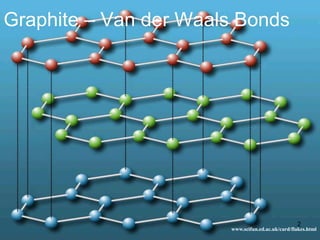
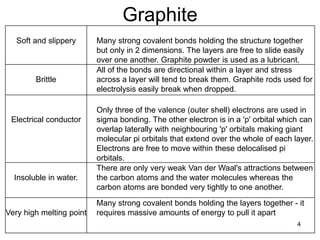
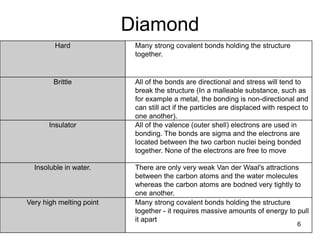
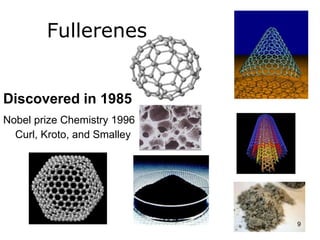
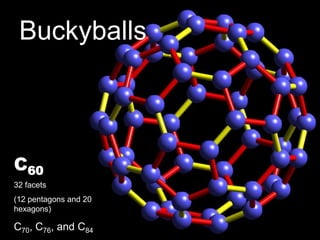
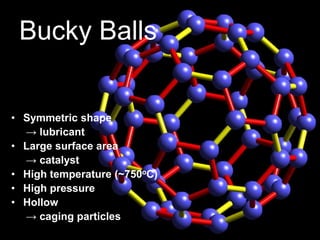
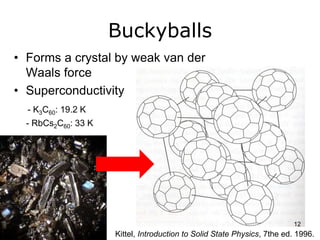
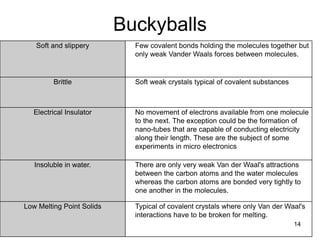
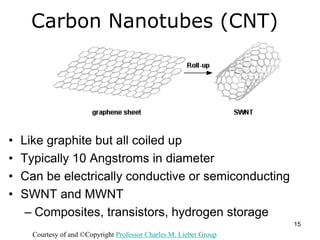
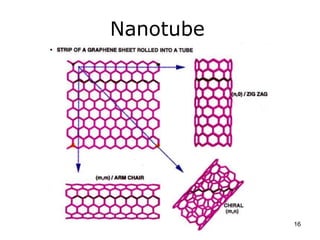
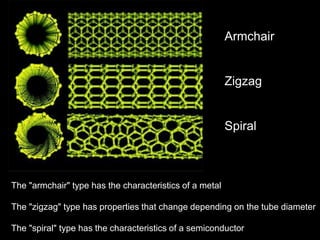
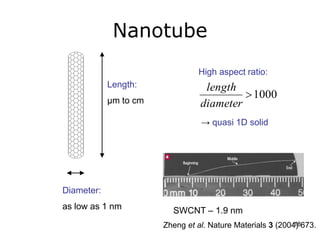
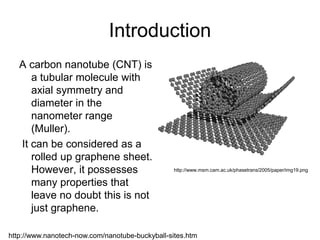
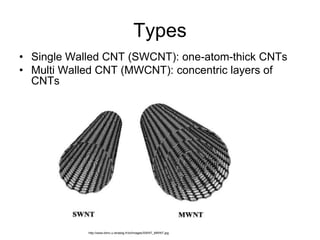
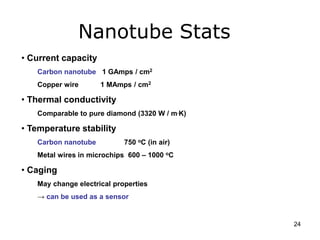
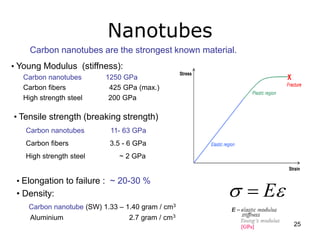
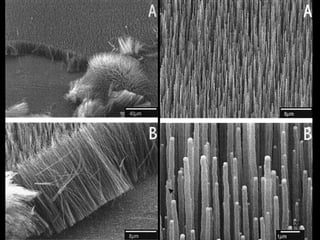
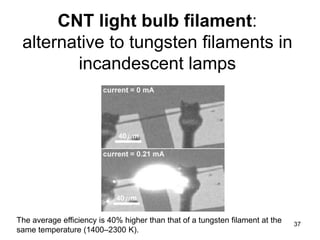
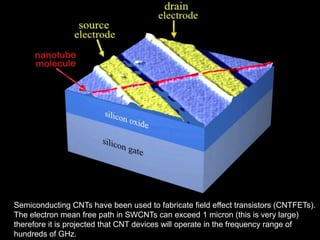
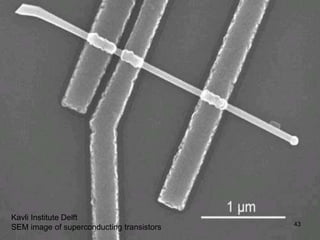
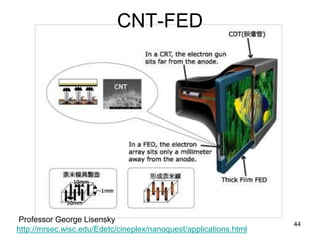
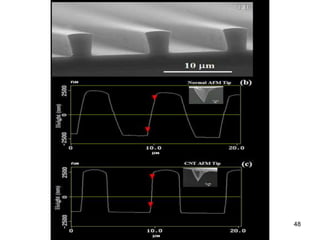
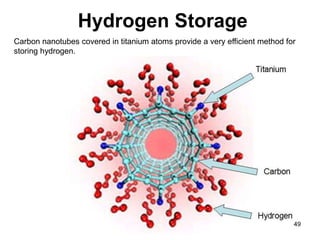
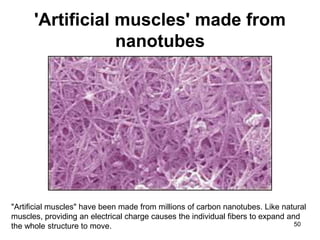
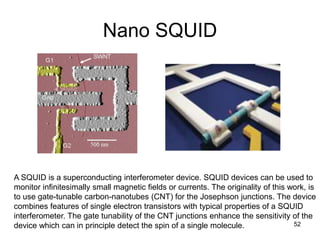
This document provides information on the properties and applications of various nanocarbon materials, including graphite, diamond, buckyballs, carbon nanotubes, and their uses. It discusses how graphite has layered structures held together by van der Waals bonds, making it soft, slippery, and electrically conductive. Diamond has a 3D covalent structure making it very hard but also brittle and an electrical insulator. Buckyballs are spherical carbon molecules that can form weak van der Waals crystals. Carbon nanotubes can be either metallic or semiconducting depending on their structure, and have excellent mechanical, thermal and electrical properties leading to uses like conductive composites and transistors.