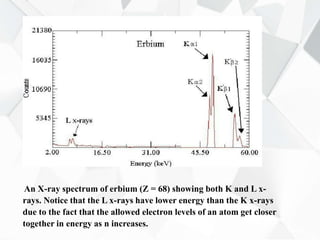
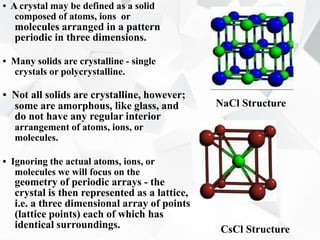
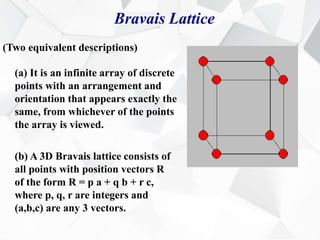
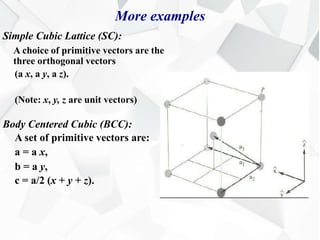
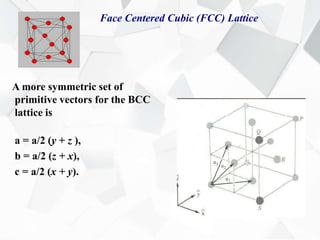
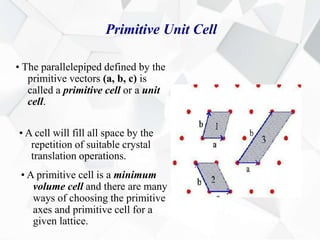
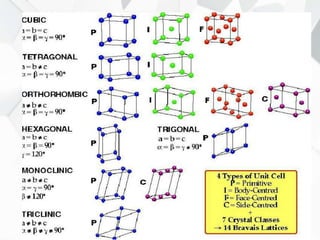
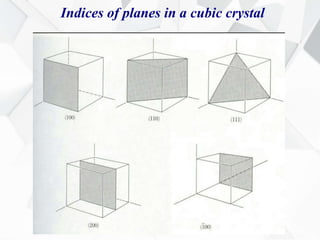
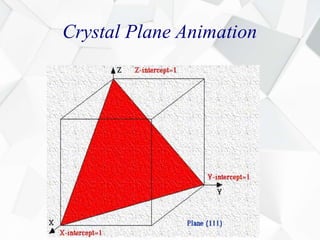
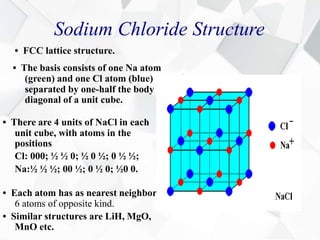
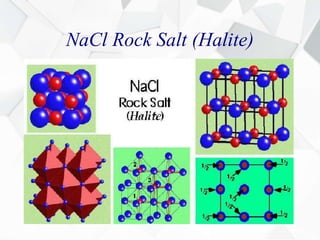
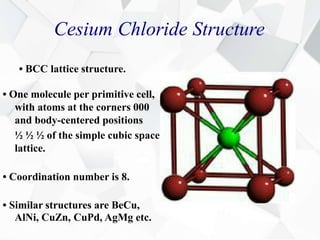
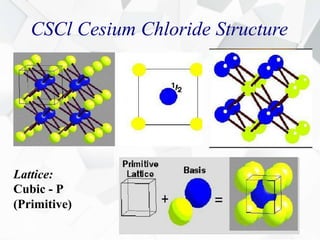
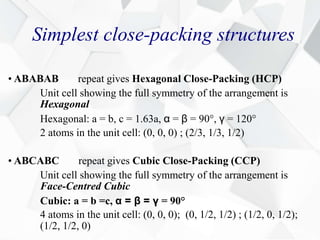
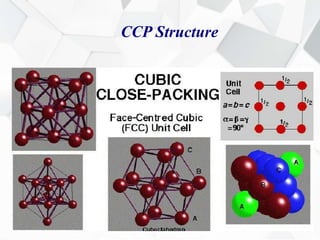
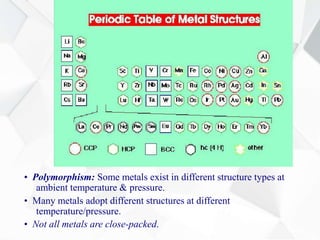
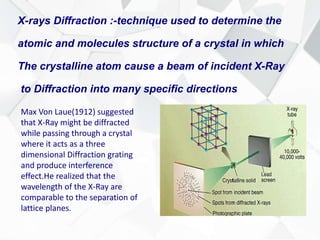
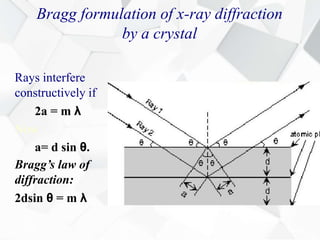
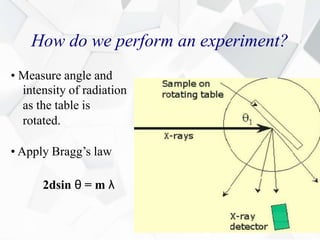
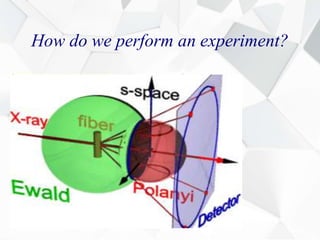
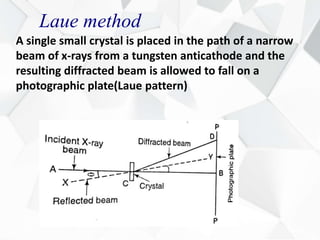
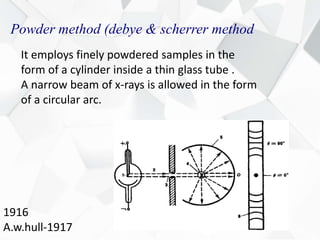
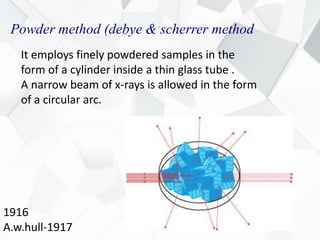
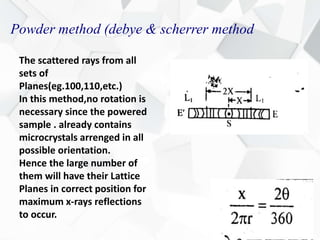
X-ray diffraction is a technique used to determine the atomic structure of crystals. When X-rays hit the periodic lattice of atoms in a crystal, the X-rays diffract into specific directions. This was first discovered by Max von Laue in 1912. Bragg's law quantifies the conditions under which constructive interference, and therefore diffraction, occurs for waves scattered by atomic planes in a crystal. X-ray diffraction methods are now commonly used to analyze crystal structures.