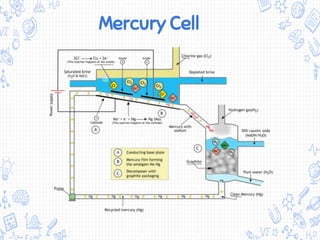
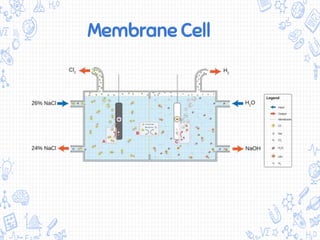
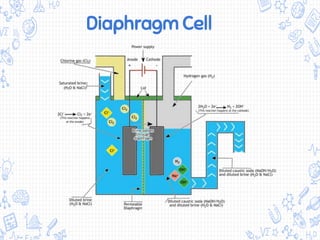
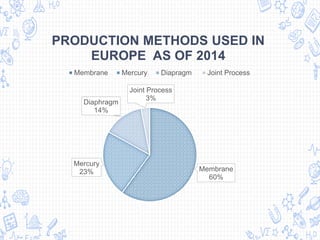
The chlor-alkali process is an industrial process that uses electrolysis to produce chlorine, sodium hydroxide, and hydrogen from salt water. It involves passing an electric current through a brine solution to drive the following reaction: 2NaCl + 2H2O → 2NaOH + Cl2 + H2. The process was first developed in the 1850s but improved in the 1890s with the mercury cell. Today, membrane and diaphragm cells are more commonly used, accounting for 60% and 14% of European production respectively. The main uses of the products are in polymers, pesticides, antiseptics, acid production, metallurgy, and the paper industry.