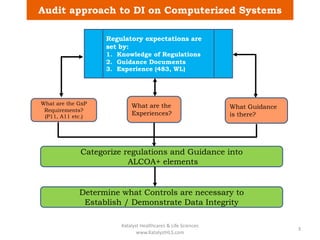
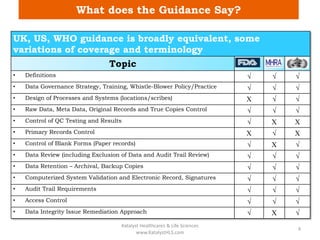
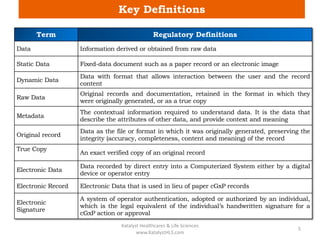
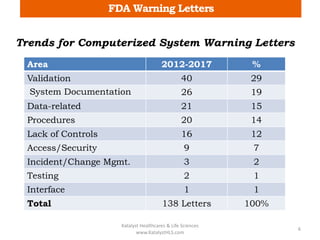
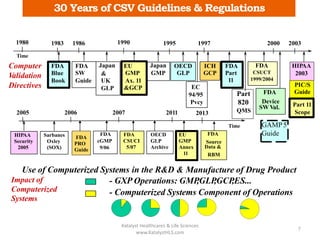
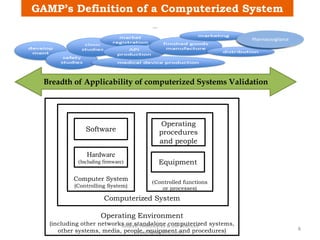
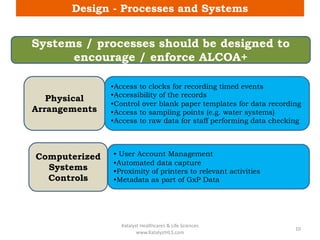
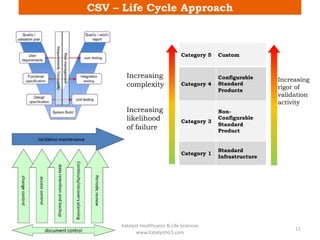
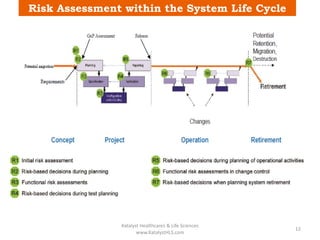
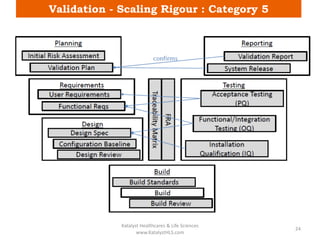
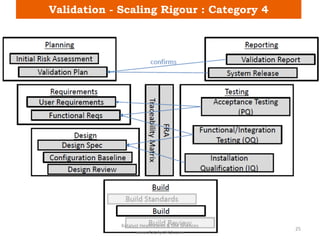
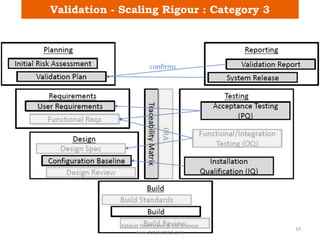
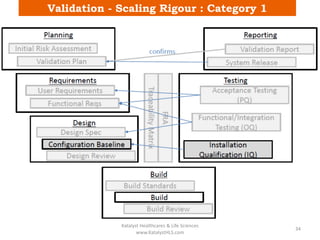
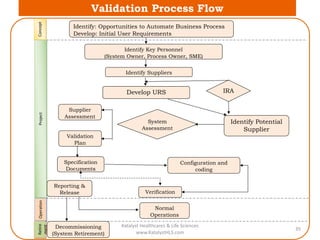
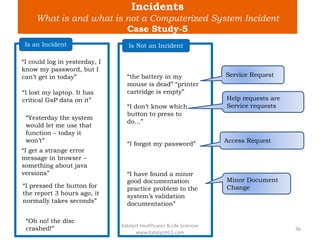
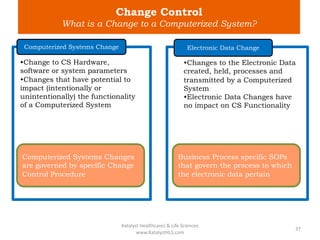
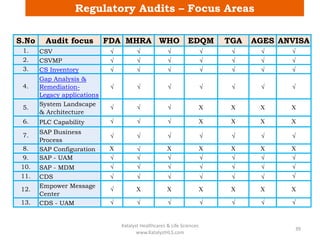
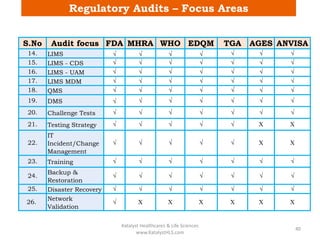
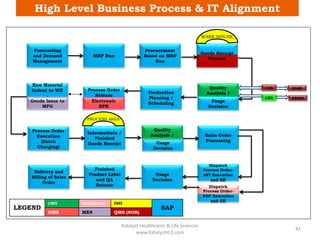
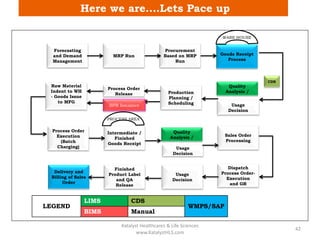
The document discusses computerized system validation (CSV) and its importance for maintaining data integrity in electronic records, highlighting regulatory expectations and guidance from authorities such as the FDA, WHO, and EMA. It covers key definitions, validation processes, risk assessment, and the life cycle of computerized systems while emphasizing the need for rigorous documentation and testing to meet regulatory compliance in good manufacturing, clinical, and laboratory practices. Additionally, it outlines focus areas for regulatory audits and the evolution of CSV guidelines over time.