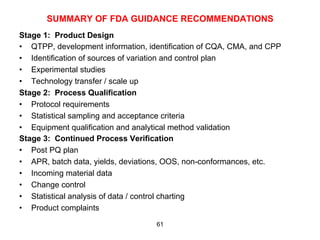
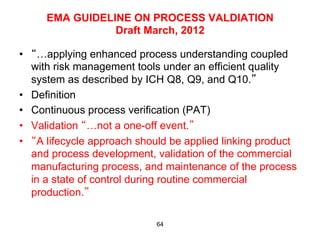
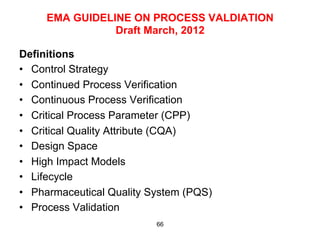
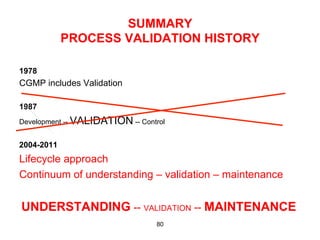
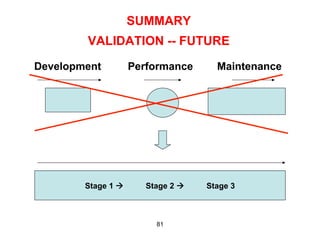
This document outlines the key concepts and history of the process validation lifecycle approach. It discusses the development of the approach through various regulatory guidances from organizations like FDA, Health Canada, EMA, ICH, and PIC/S. The lifecycle approach involves three stages: process design, process qualification, and continued process verification. It represents a shift from a traditional approach of conducting process validation to one focused on continual process improvement and understanding over the entire product lifecycle. Implementation of the approach can be difficult for organizations.