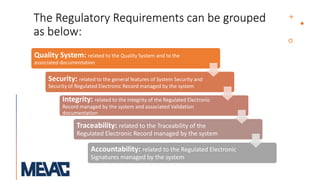
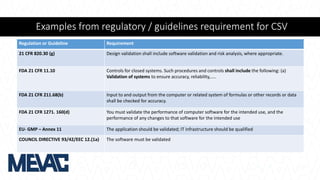
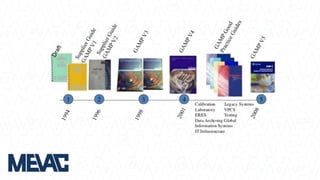
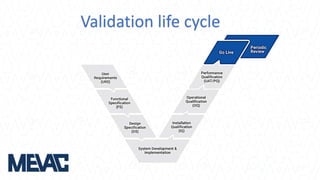
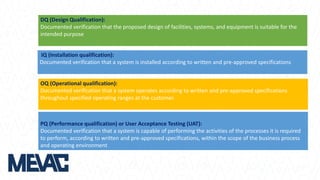
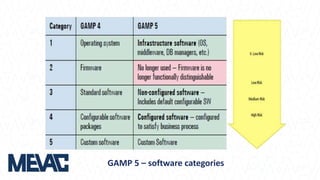
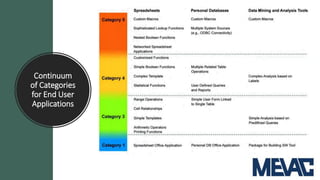
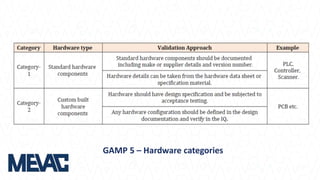
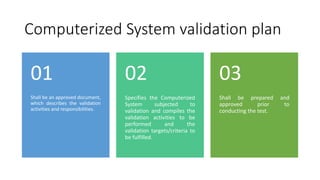
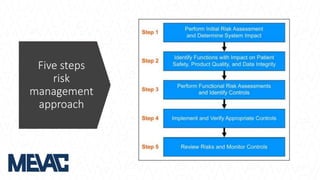
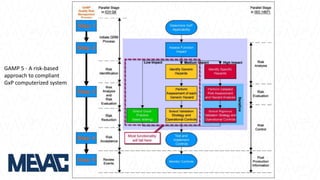
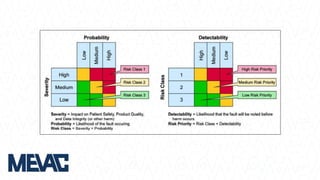
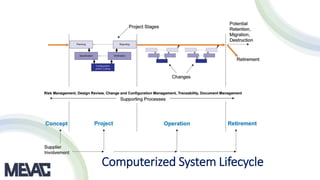
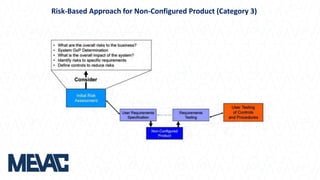
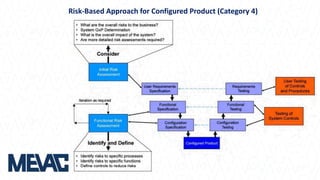
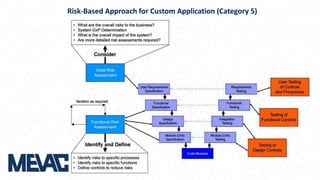
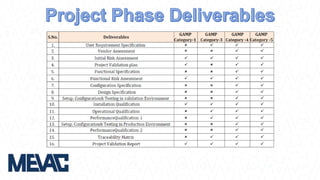
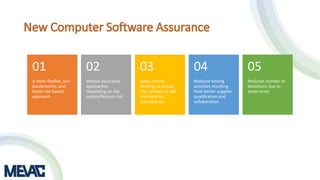
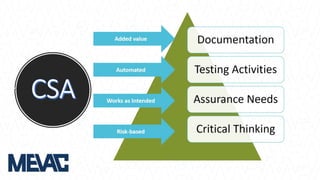
This document discusses Computerized System Validation (CSV) as a critical requirement for Good Manufacturing Practices (GMP), emphasizing the importance of validation in ensuring product quality and regulatory compliance. It outlines the regulatory framework, benefits of CSV, and the lifecycle of validation processes including user requirements, performance qualification, and risk management. The document also highlights the transition from CSV to Computer Software Assurance (CSA) as a future approach for software acceptance in the quality management field.