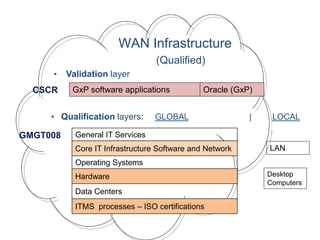
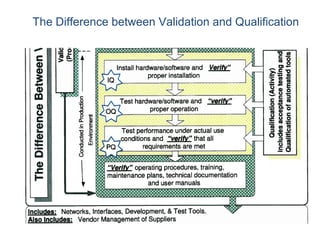
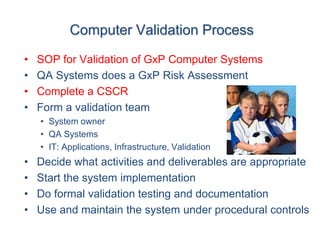
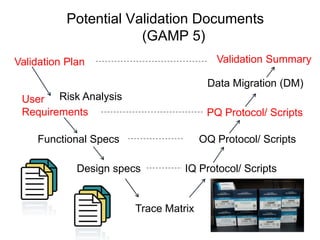
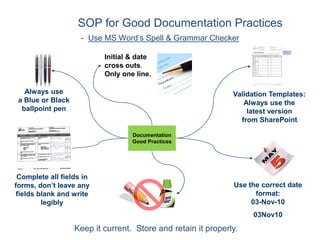
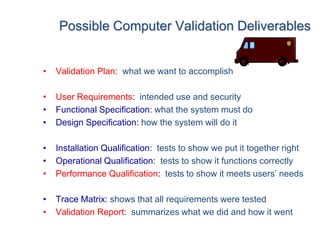
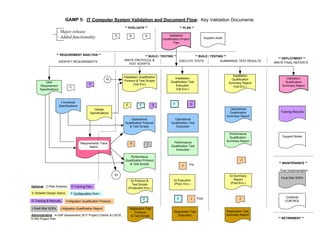
This document outlines the importance of IT validation in ensuring that software and systems meet specified requirements and regulatory standards, particularly those set by the FDA. It details the validation process, key practices, documentation requirements, and the implications of validation on patient safety and product quality. The document also emphasizes the need for ongoing procedural controls, change management, and regular evaluations to maintain system compliance and effectiveness.