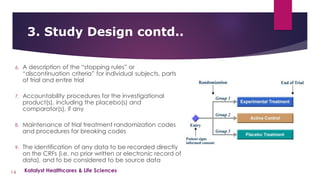
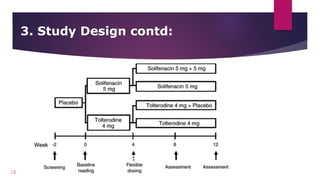
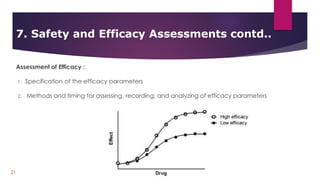
The document provides an overview of clinical study protocols, including their purpose, key components, and importance. It describes the various sections of a protocol, such as the background and objectives, study design, subject selection criteria, study procedures, safety and efficacy assessments, and data handling. Maintaining adherence to the protocol is important to ensure the safety of participants and integrity of the clinical trial.