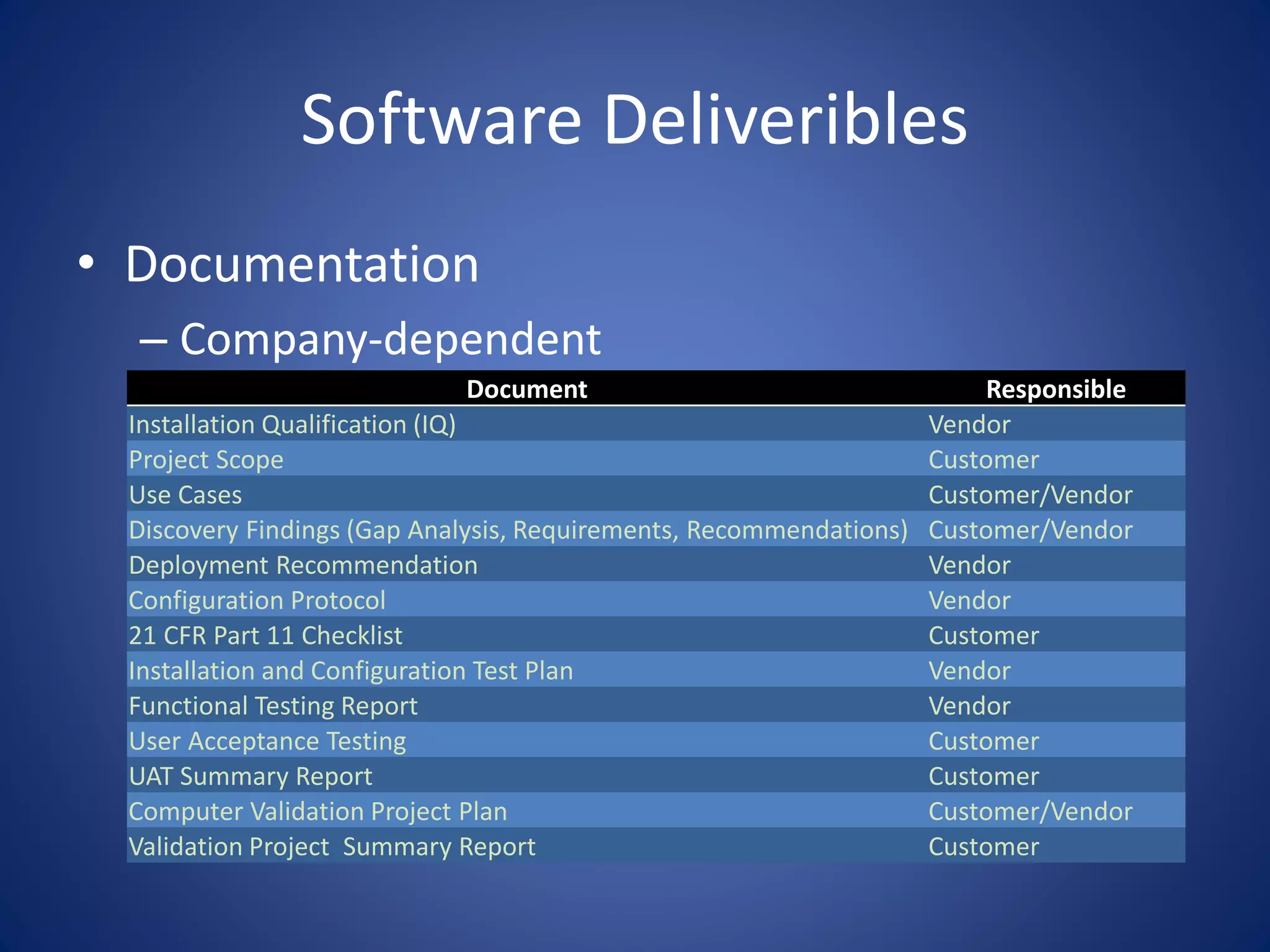
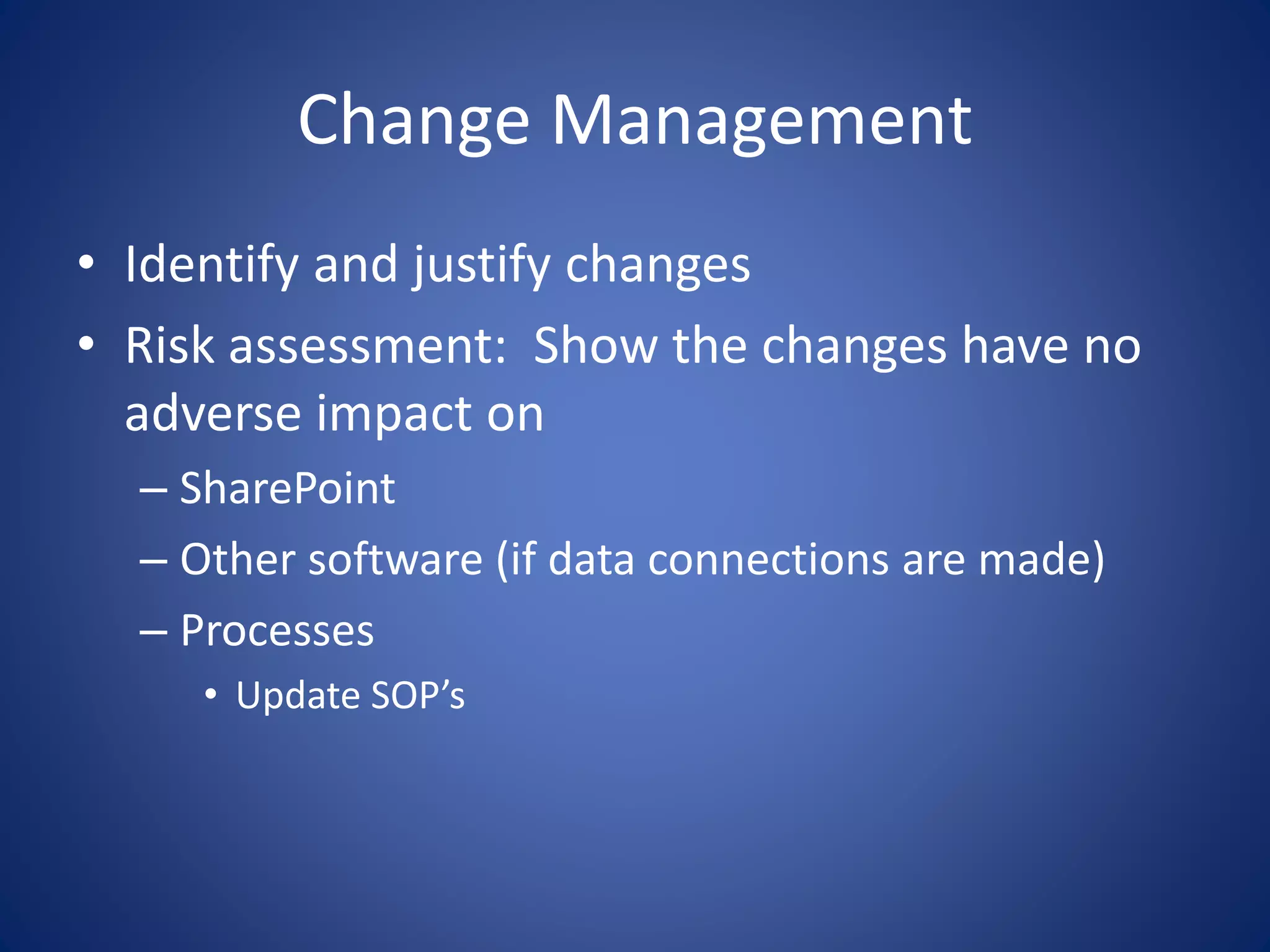
This document discusses providing SharePoint solutions in a regulated FDA environment. It provides background on the FDA and drug approval process. It explains that computer systems used for clinical trials and drug development must be validated per 21 CFR Part 11. SharePoint can be used, but requires configuration in a separate isolated environment, change control, and validation documentation to ensure it meets regulatory requirements as an electronic records system. Documentation of installation, testing, and ongoing system management is necessary to comply with FDA regulations.