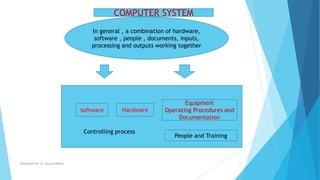
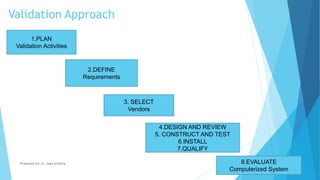
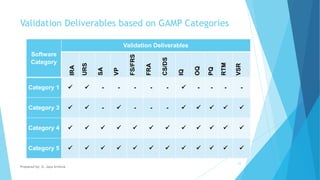
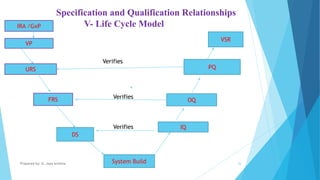
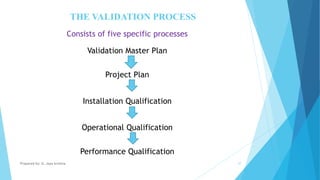
The document discusses the importance of Computerized System Validation (CSV) in the pharmaceutical industry, highlighting the regulatory focus on data integrity due to serious non-compliance issues triggering regulatory actions. It outlines the validation process, types of validation, and the GAMP5 guidelines, which provide a framework for ensuring software quality and compliance. It emphasizes that non-compliance can lead to severe financial consequences and operational risks, underscoring the need for well-documented validation processes.