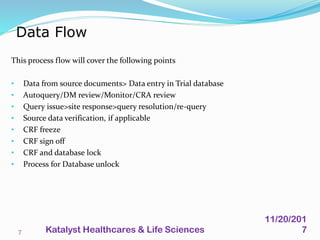
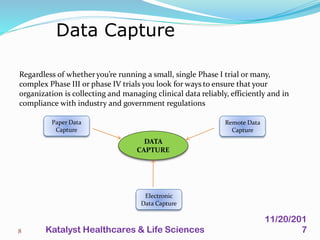
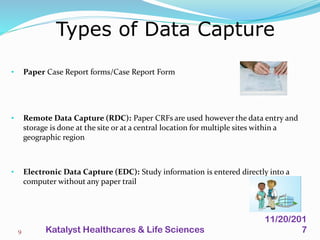
A data management plan (DMP) ensures consistent and effective clinical data management practices throughout a clinical trial. The DMP describes all data management activities, responsibilities, and deliverables to promote agreement among parties. It not only describes the data management process, but also provides documentation of data handling for regulatory compliance. The DMP components include data flow, capture, setup, entry, transfer, processing, coding, safety data, external data, database locking and unlocking, archiving, and quality reports. It serves to plan, communicate, and reference all data management tasks during a study.