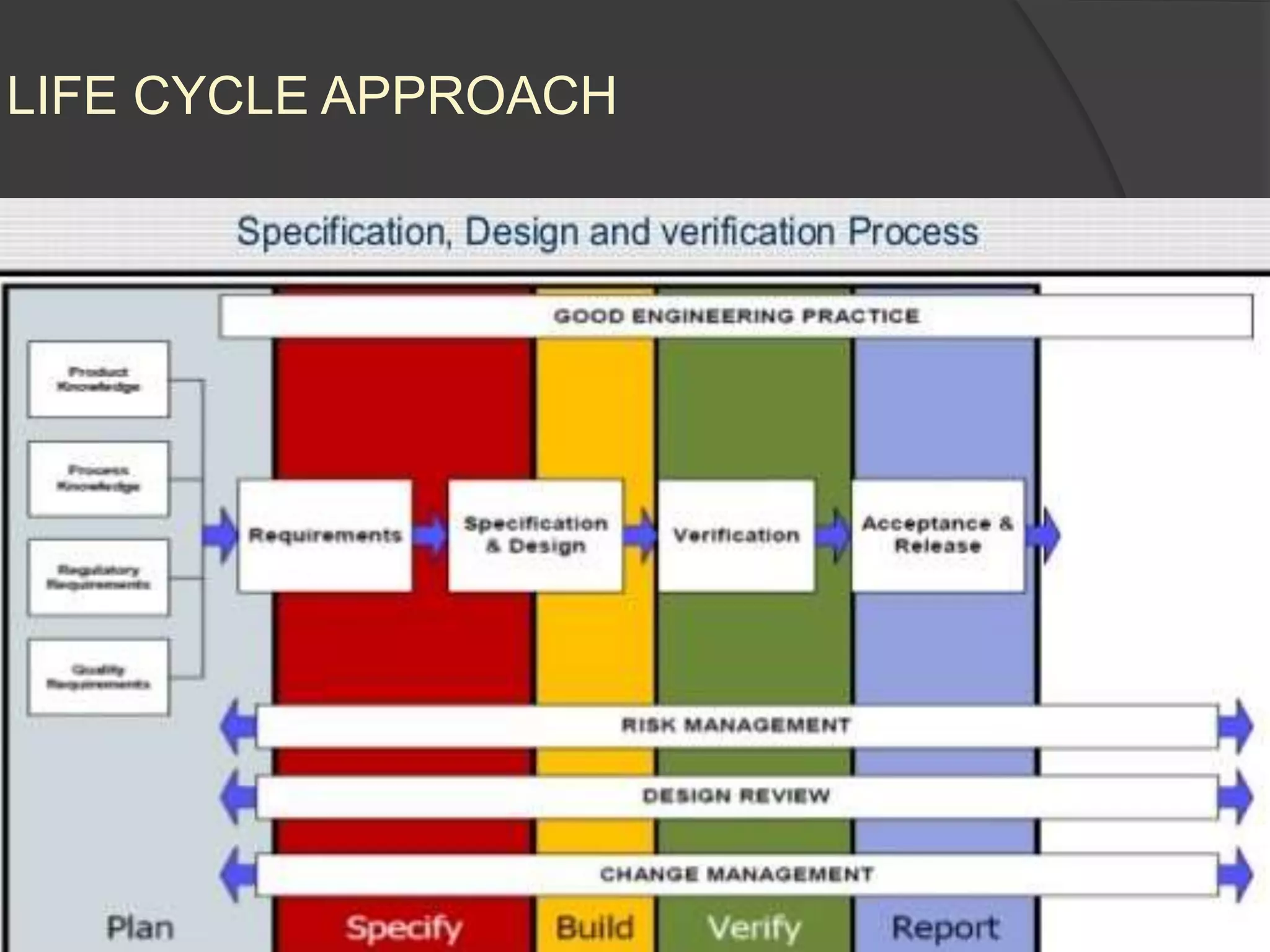
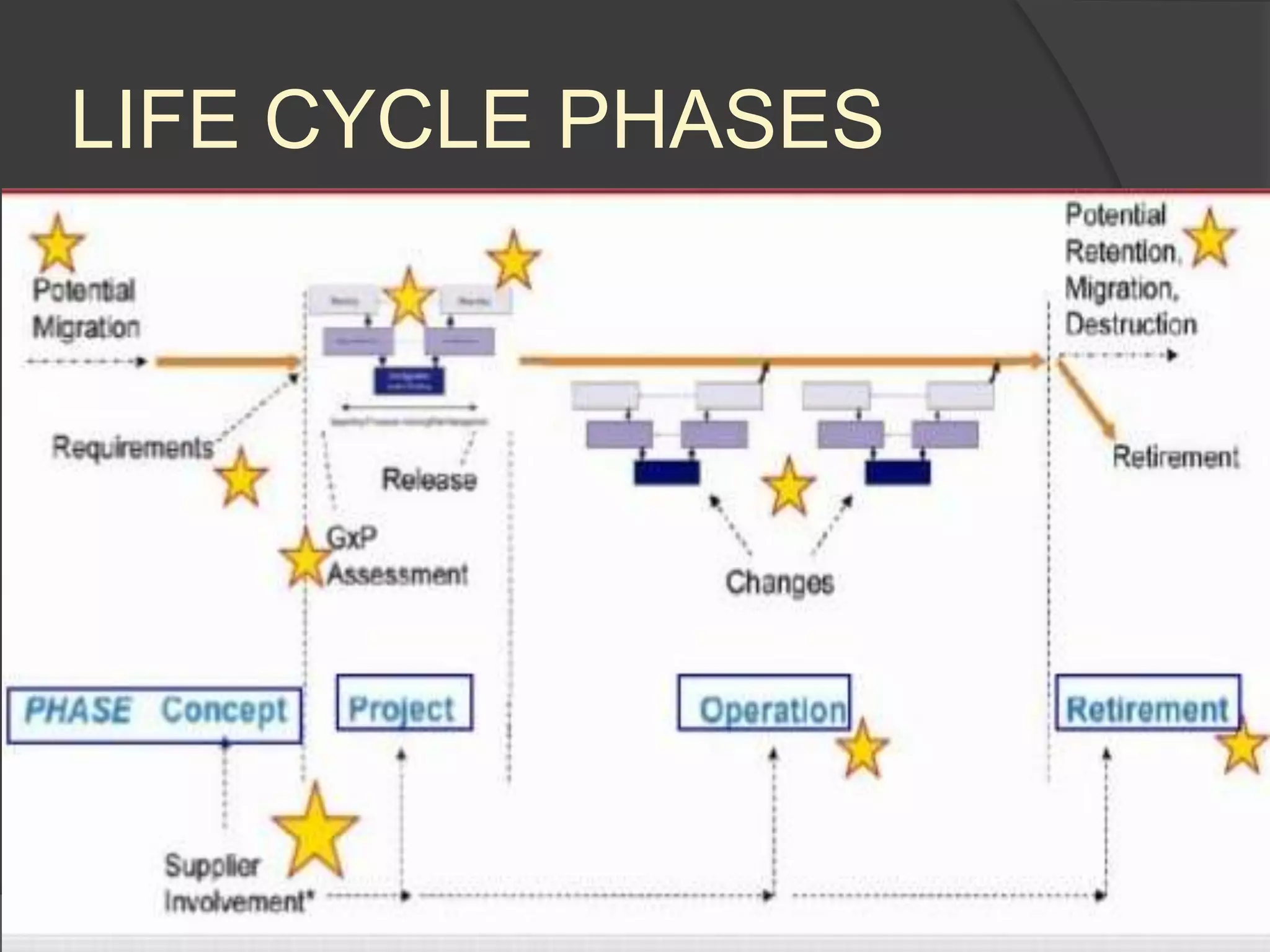
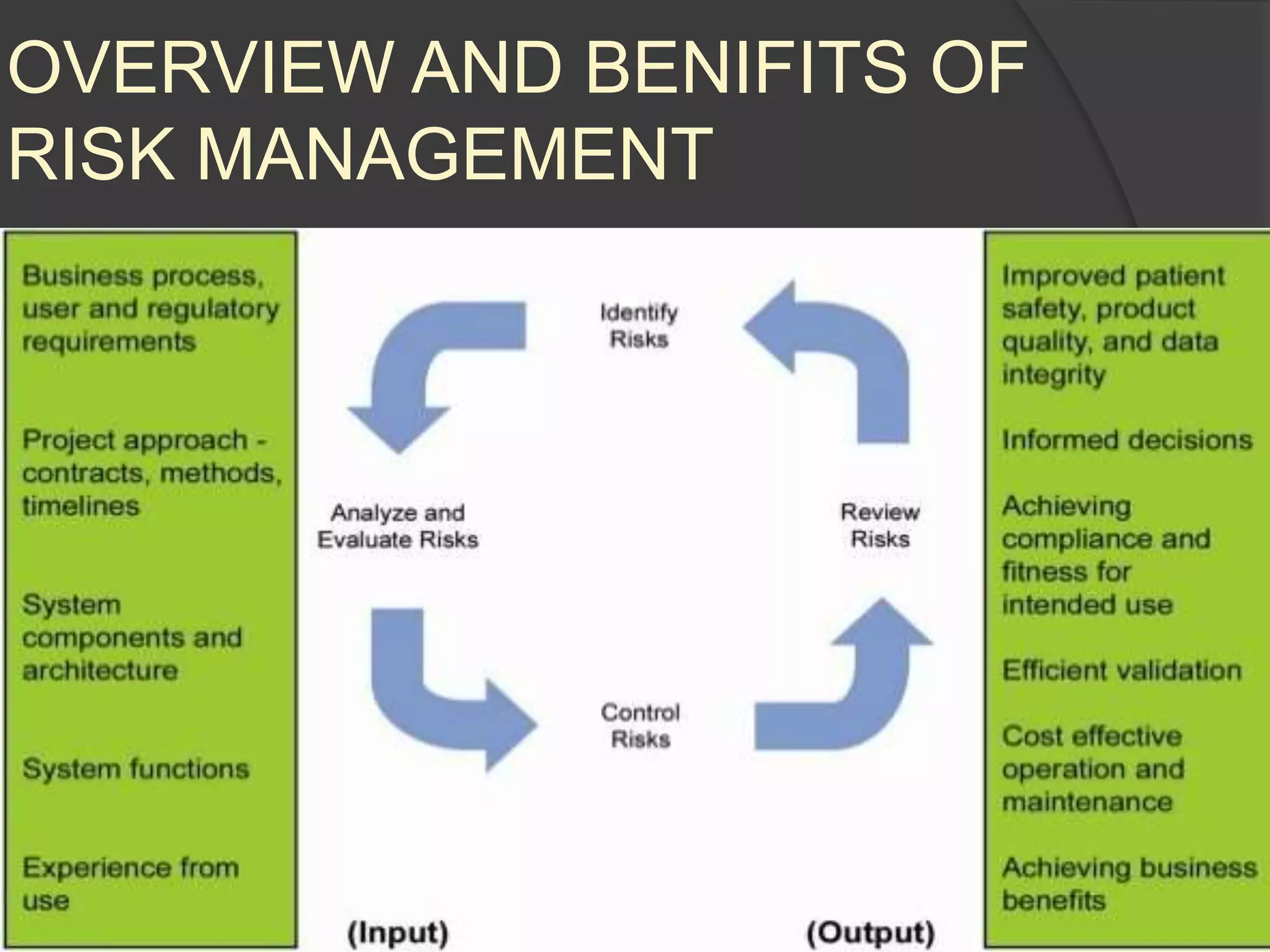
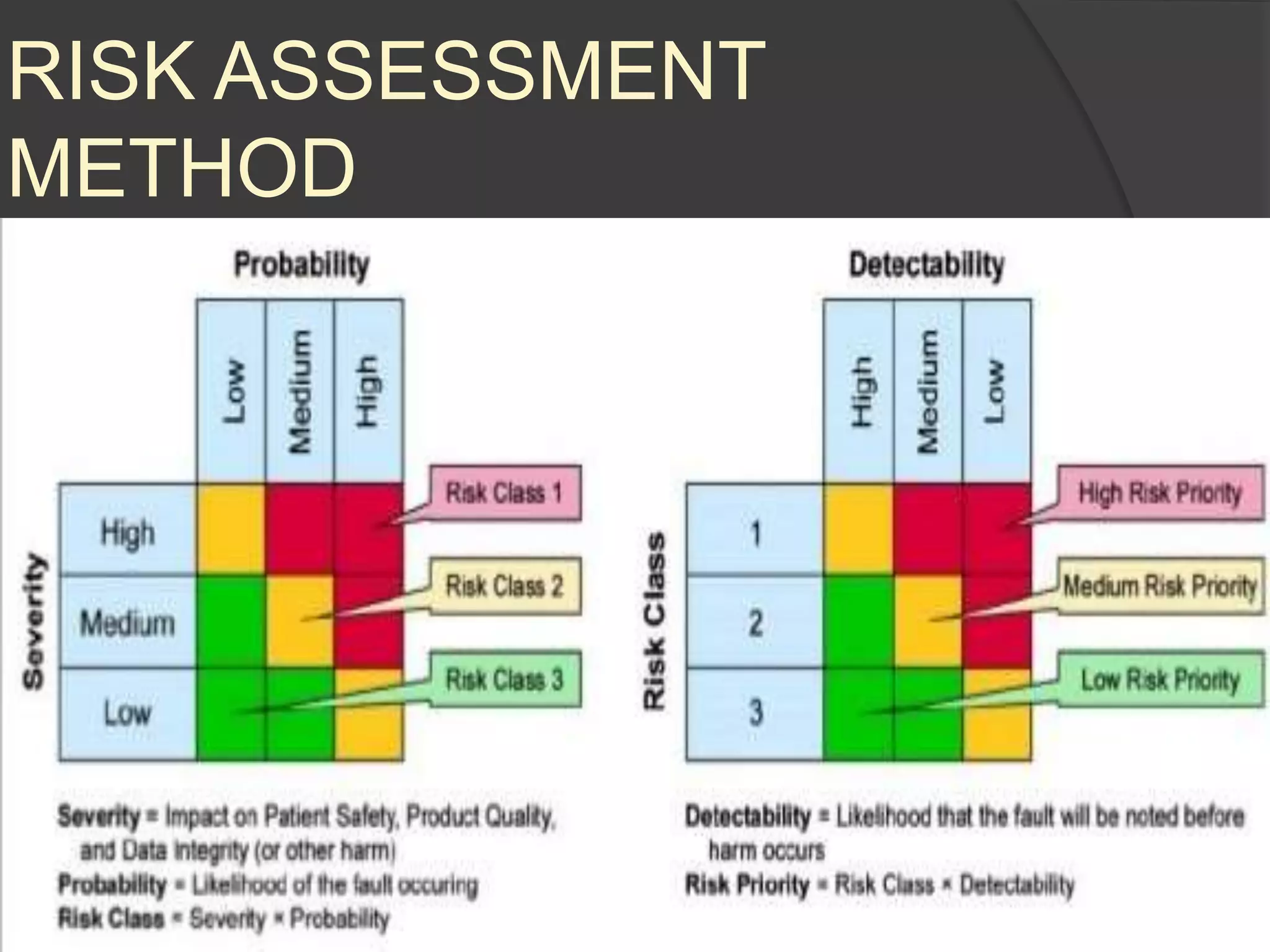
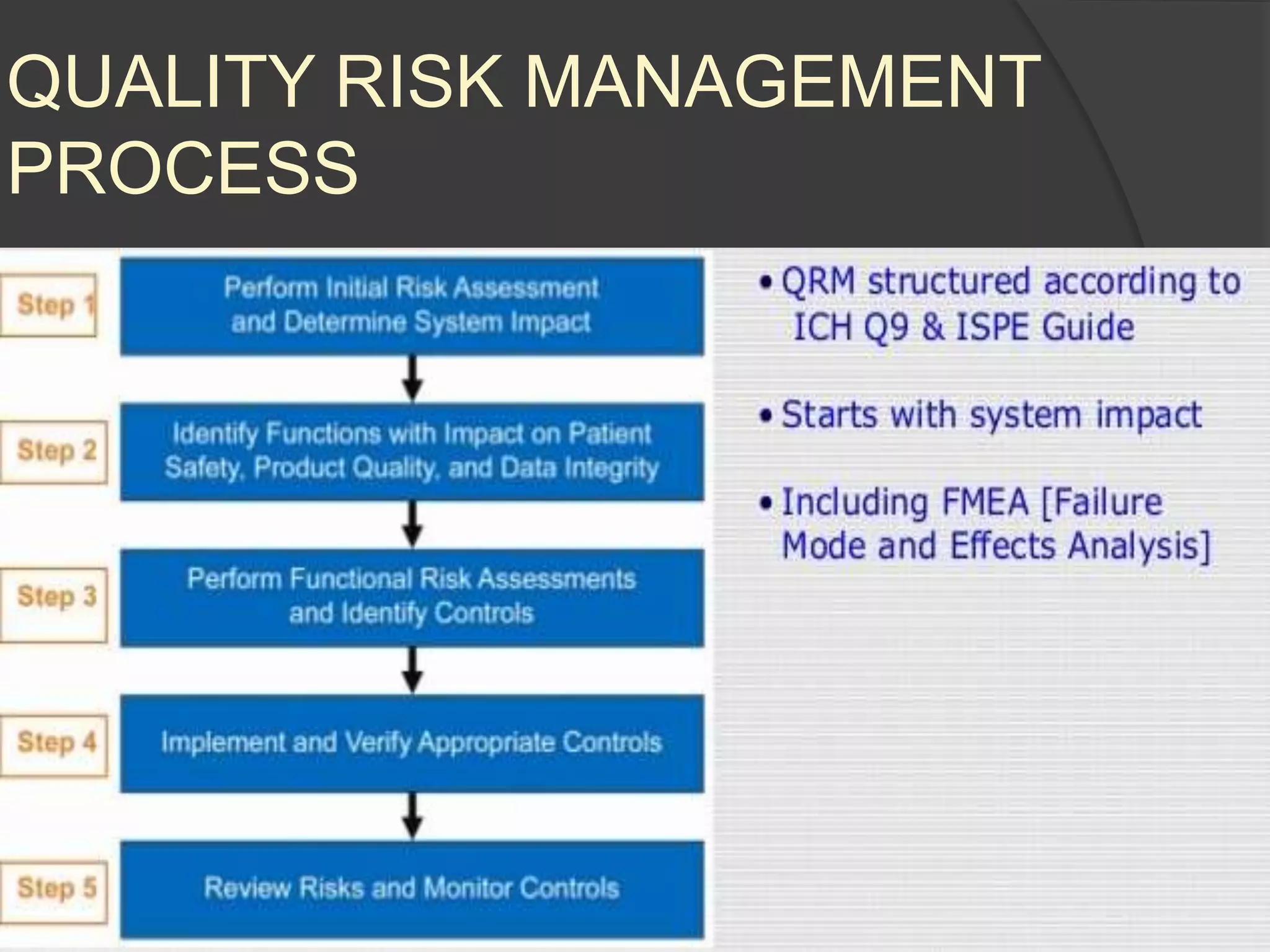
GAMP 5 provides a framework for validating computerized systems used in regulated industries. It recommends a life cycle approach involving quality risk management throughout planning, development, validation and operation. Key activities for regulated companies include governance, identifying systems' impact, and ensuring compliance. Suppliers play an important role by providing documentation, testing systems, and supporting changes and maintenance. The level of validation should be based on a system's risk, complexity and novelty.