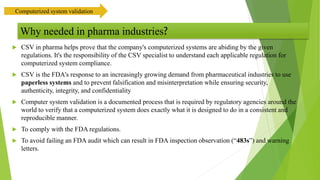
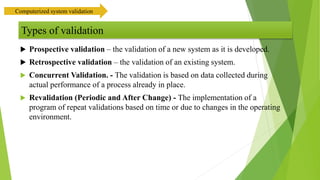
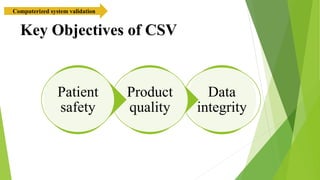
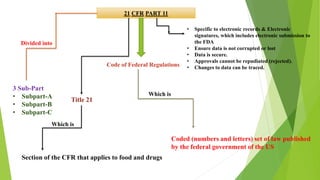
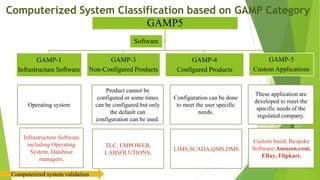
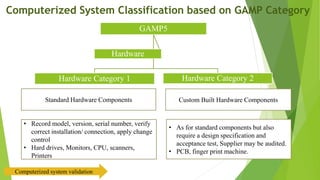
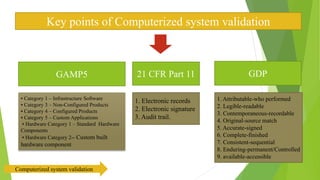
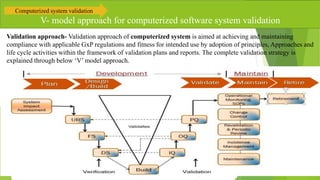
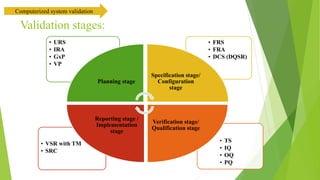
The document provides an overview of computerized system validation. It defines computerized system validation as the process of testing, validating, and qualifying a regulated computerized system to ensure it operates as designed in a consistent and reproducible manner. The document discusses the difference between computer systems and computerized systems, why validation is needed in the pharmaceutical industry, types of validation, applicable regulatory requirements like 21 CFR Part 11, and the GAMP 5 categories for classifying computerized systems. It provides key points about computerized system validation and the V-model approach for validation stages and deliverables.