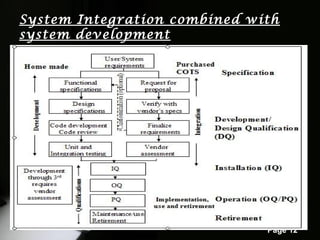
The document outlines computer system validation (CSV) as a process to ensure that computer systems consistently produce results that meet predetermined specifications and quality attributes. It details various steps in the validation process, including planning, user requirements, vendor assessment, and different CSV models, along with specific examples such as HPLC software validation. The importance of risk assessment and ongoing validation throughout the system lifecycle is emphasized, alongside guidelines for effective vendor assessment.