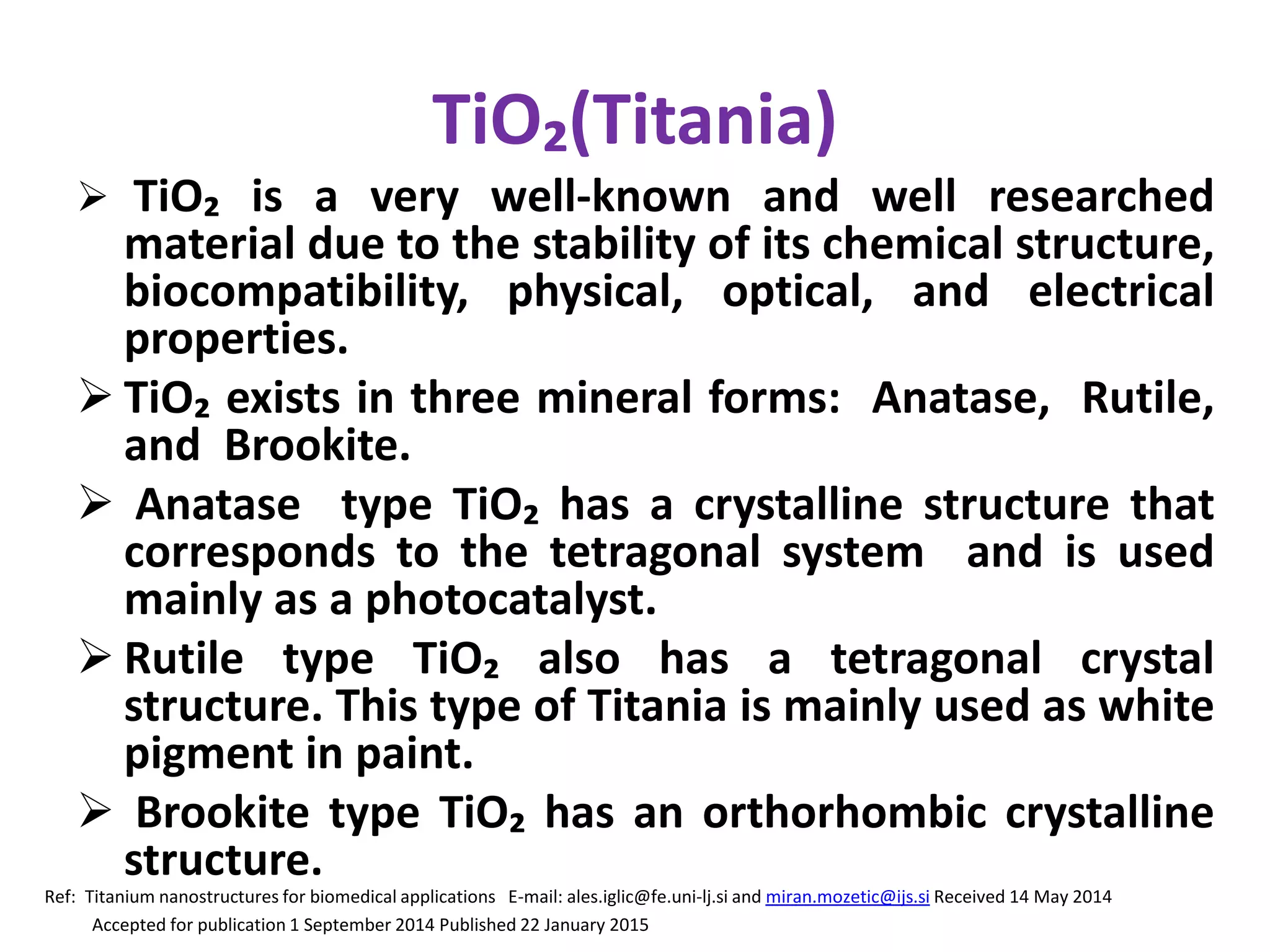
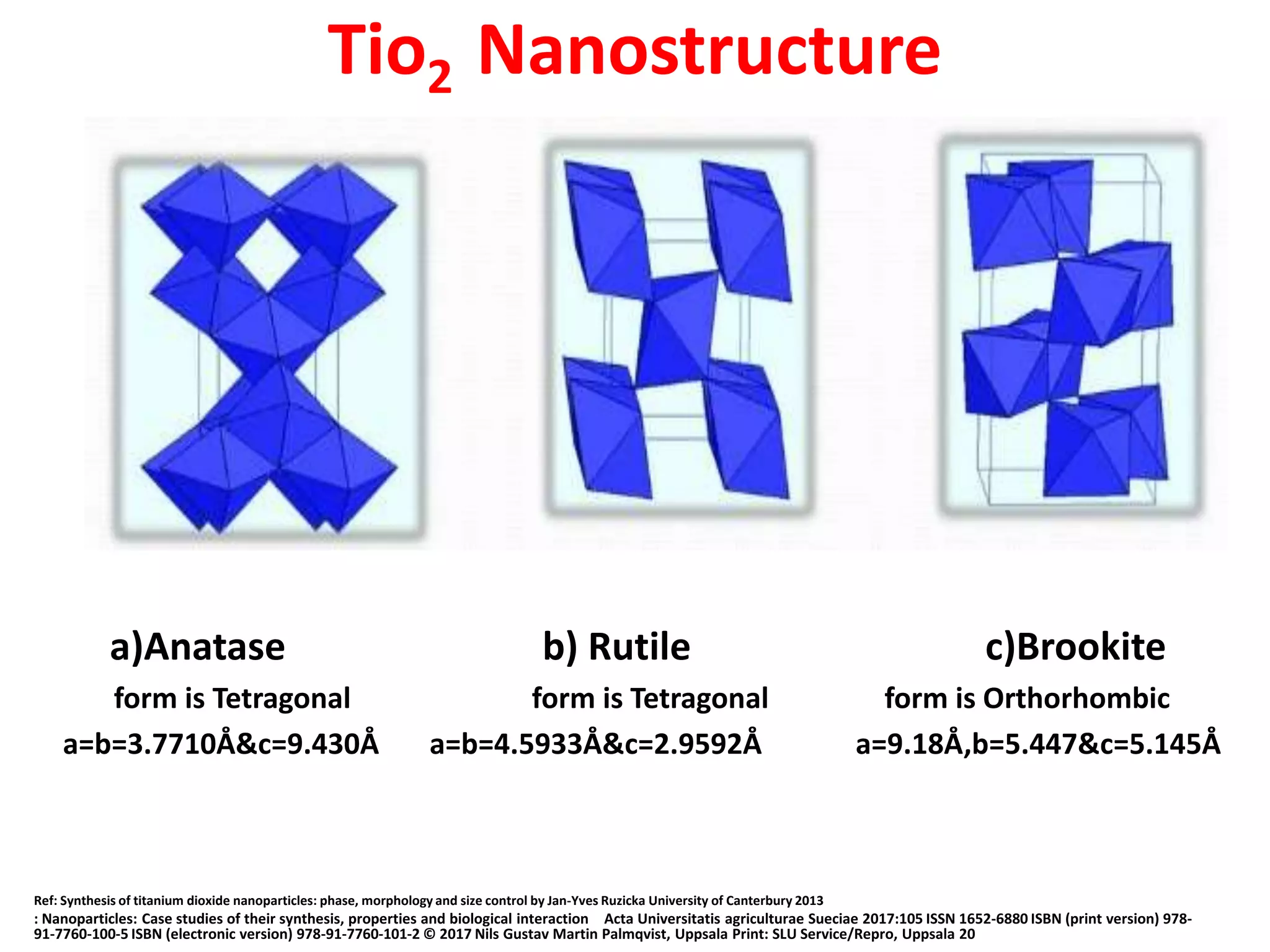
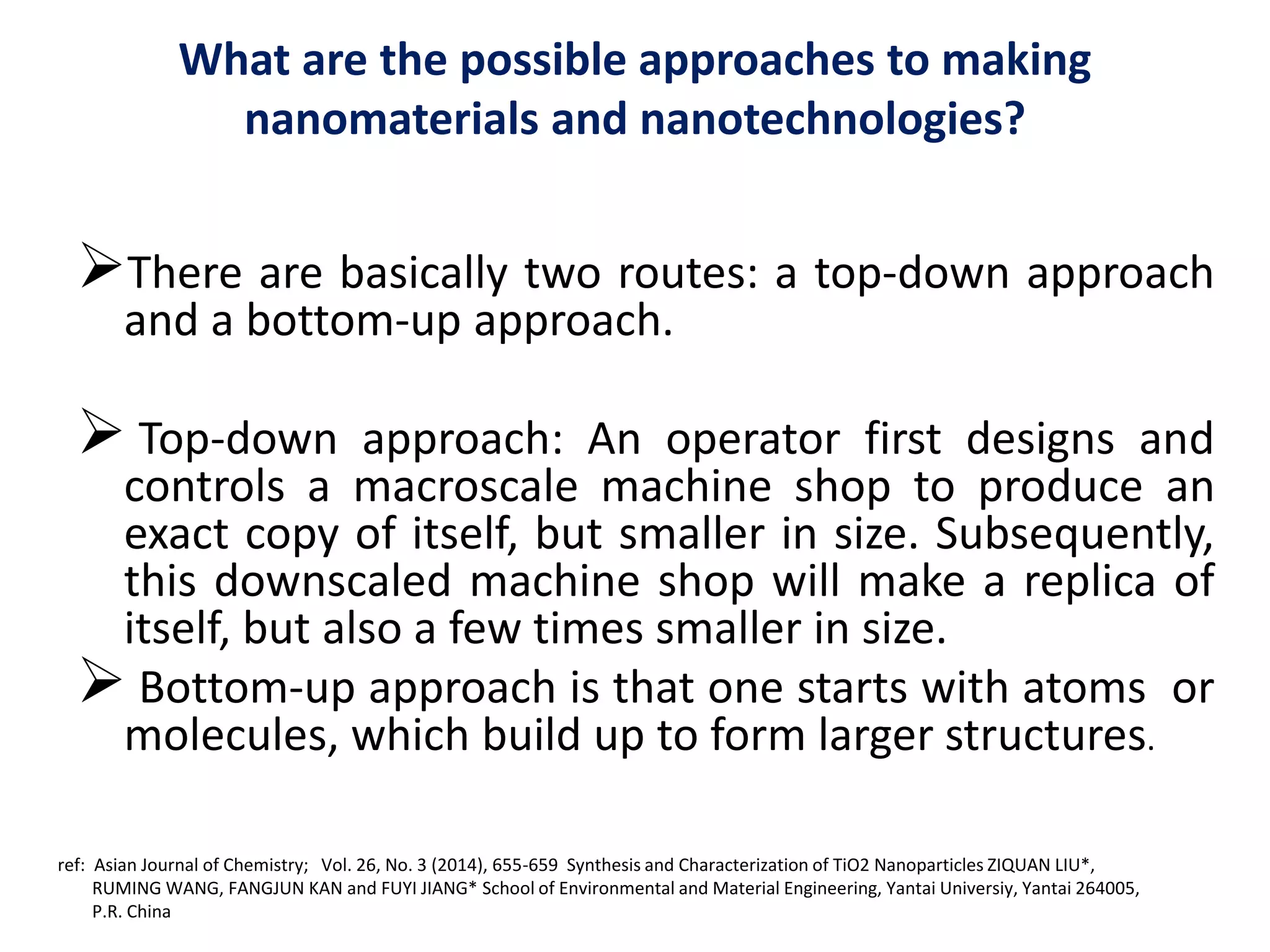
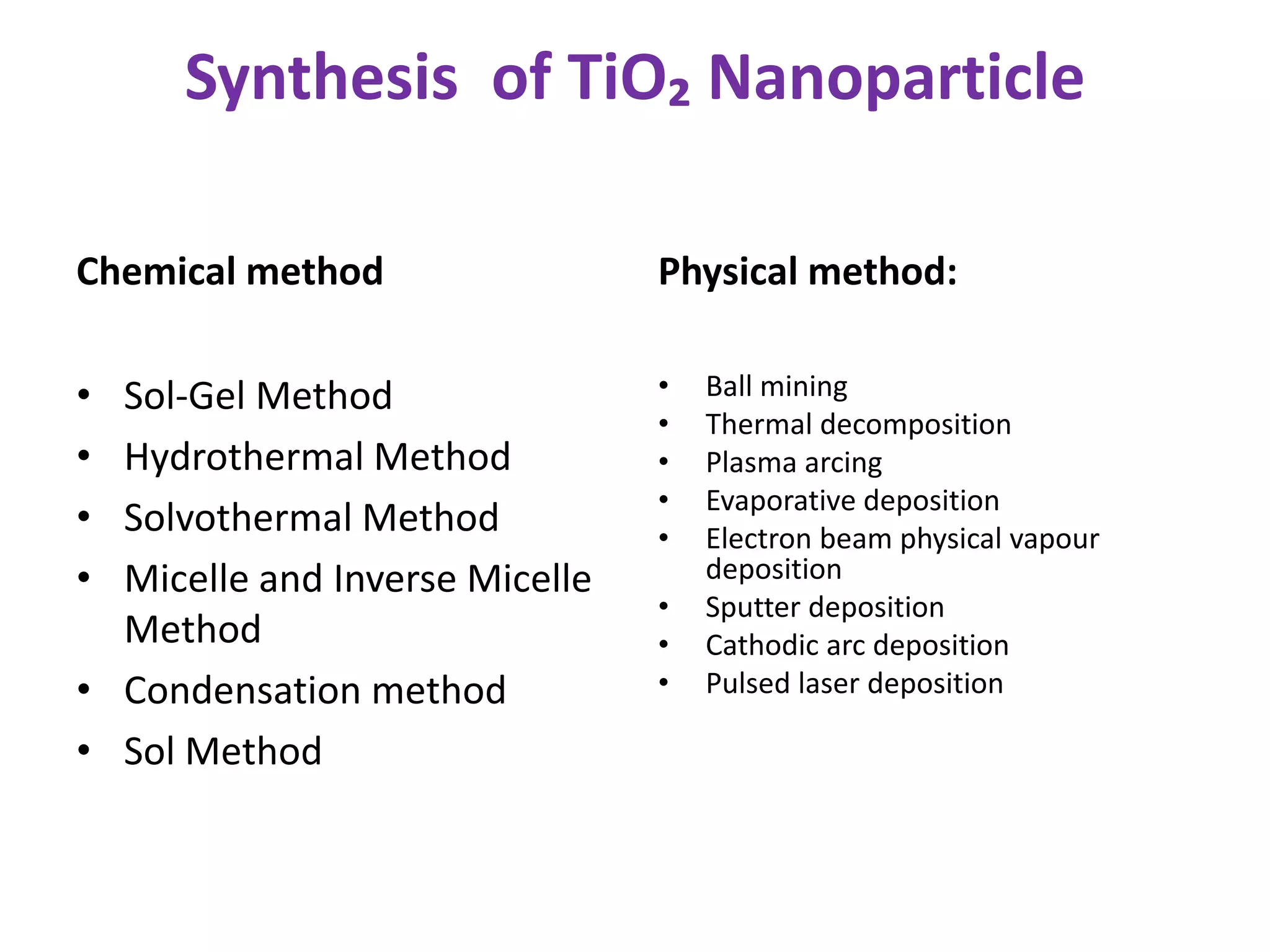
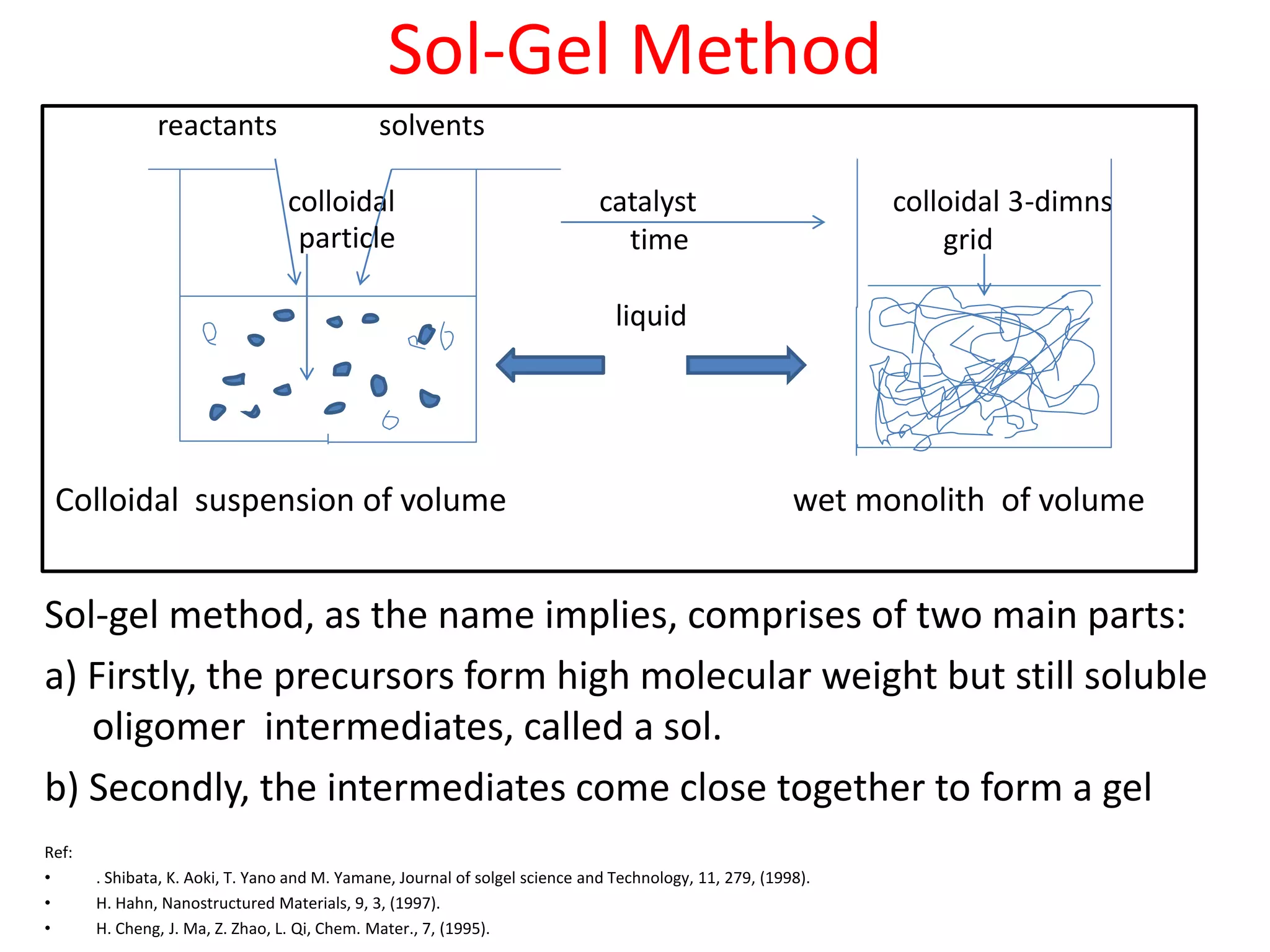
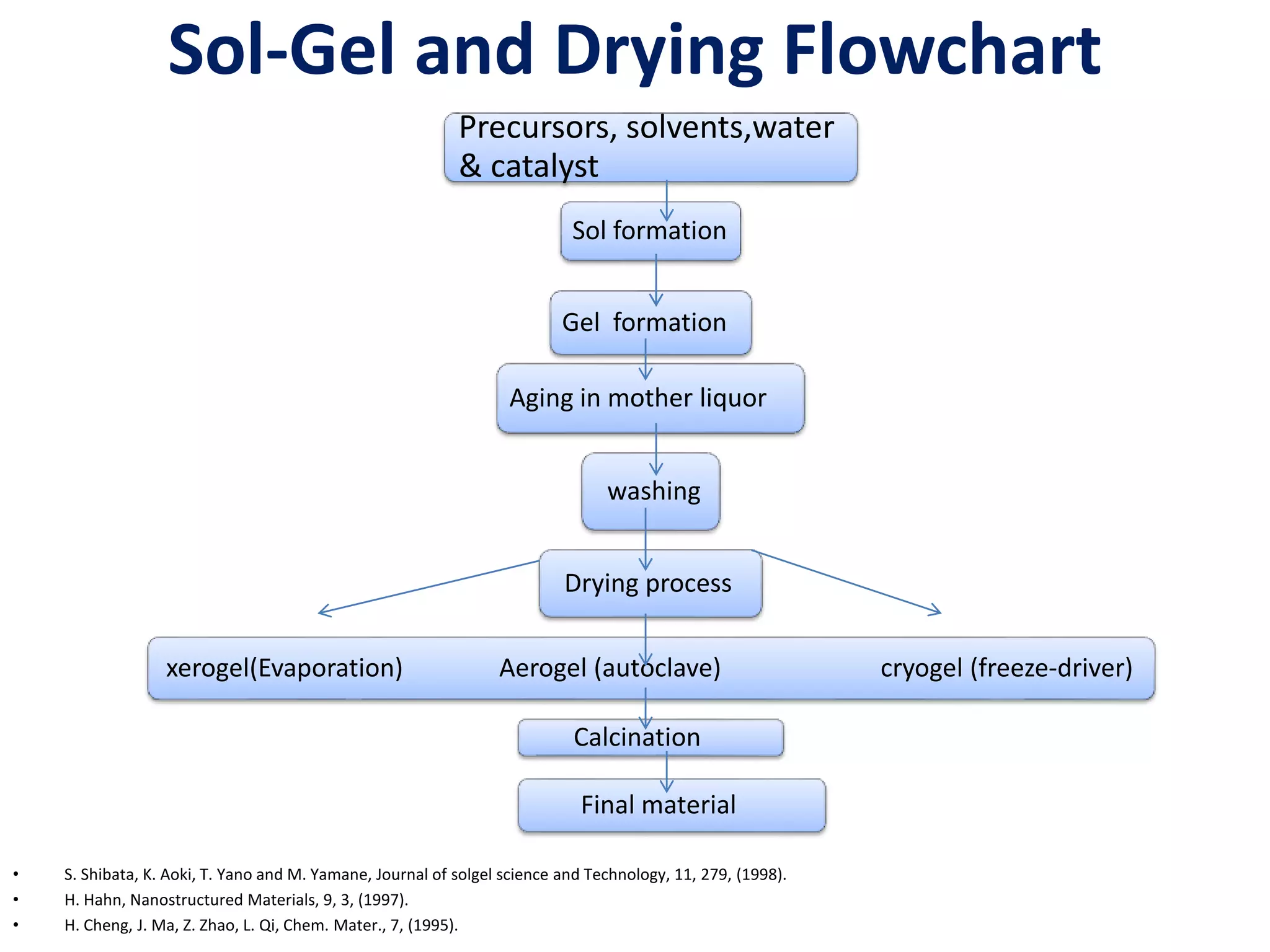
The document is a review and investigation of titanium dioxide (TiO2) nanostructures for biomedical applications, detailing its properties, synthesis methods, and characterization techniques. It discusses the various forms of TiO2, primarily anatase, rutile, and brookite, and their applications in areas such as drug delivery, photodynamic therapy, and antimicrobial agents. Future plans include synthesizing TiO2 via the sol-gel method and applying it in biomedical contexts.